Summary
This article discusses results from the late-breaking Study Comparing the Effect on Disease Activity When Reducing or Discontinuing Etanercept in Subjects with RA [DOSERA; NCT00858780]. The results showed that in patients with rheumatoid arthritis and stable low disease activity on methotrexate plus etanercept, continued treatment with etanercept at 25 or 50 mg/week provides a significantly higher likelihood of maintaining a stable disease state over 48 weeks than placebo.
- Rheumatology Clinical Trials
- Rheumatoid Arthritis
Results from the late-breaking Study Comparing the Effect on Disease Activity When Reducing or Discontinuing Etanercept in Subjects with RA [DOSERA; NCT00858780] were reported by Ronald F. van Vollenhoven, MD, Karolinska Institute, Stockholm, Sweden. The results showed that in patients with rheumatoid arthritis (RA) and stable low disease activity on methotrexate plus etanercept, continued treatment with etanercept at 25 or 50 mg/week provides a significantly higher likelihood of maintaining a stable disease state over 48 weeks than placebo. Discontinuation of etanercept leads to worsening.
Etanercept has been shown to have sustained efficacy over 3 years, and it has a favorable safety profile [Klareskog L et al. Ann Rheum Dis 2006]. Its efficacy in combination with methotrexate in the treatment of RA is well established [Rexhepi S et al. Arthritis Res Ther 2012]; however, it is not known whether etanercept must be continued to maintain low disease activity/remission (LDA/REM) or if the continuation of methotrexate alone or with a lower dose of etanercept might be equally effective.
This was a randomized, double-blind, 3-arm study conducted in 5 Northern European countries. Adult patients with RA treated with stable background methotrexate (7.5 to 25 mg/week) plus etanercept (50 mg/week) for ≥14 months, with a 28-joint Disease Activity Score (DAS28) ≤3.2 for at least 11 months were randomized (1:1:1) to methotrexate plus etanercept 50 mg/week (etanercept50), etanercept 25 mg/week (etanercept25), or placebo. The primary study outcome was the proportion of patients in the etanercept50 group who were nonfailures at 48 weeks. Failure was defined as DAS28 >3.2 and increased by 0.6 or disease progression determined by investigator or subject. Secondary outcomes included comparisons of nonfailure and DAS28 outcomes for all 3 groups, and time to failure. The primary outcome was analyzed using a Generalized Estimating Equation model and expressed as the odds ratio (OR; 95% CI) for achieving nonfailure. Patients were followed for 2 months without major changes in therapy to ensure stable LDA/REM and stratified based on LDA/REM status. Seventy-three patients were randomized, 70% were women, mean age was 57 years, and mean duration of etanercept treatment was 3.88 years. Twenty percent of subjects were in remission and 5% had low disease activity. Mean DAS28 score at the start of etanercept treatment was 5.0.
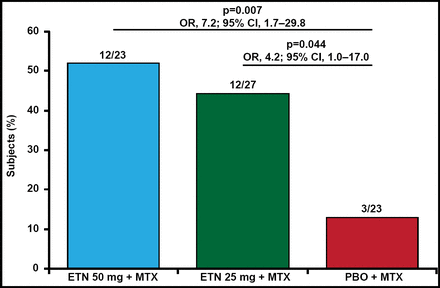
After 48 weeks, the proportion of nonfailures was 52% for etanercept50 (OR, 7.2; 95% CI, 1.7 to 29.8; p=0.007 vs placebo) and 44% for etanercept25 (OR, 4.2; 95% CI, 1.0 to 17.0; p=0.044 vs placebo; Figure 1). Median time to failure was 6 weeks from randomization for placebo, and 48 and 36 weeks for etanercept50 and etanercept25, respectively. Adverse events were similar between the groups and no unexpected safety signals were noted.
Nonfailures at Week 48.
ETN=etanercept; MTX=methotrexate; PBO=placebo.
Adapted from RF van Vollenhoven, MD.
The data suggest that induction-maintenance may be possible with etanercept for some RA patients, even in established disease.
- © 2012 MD Conference Express®