Summary
This article presents data from the British Society for Rheumatology Biologics Register, a large cohort study showing no evidence that antibody to tumor necrosis factor therapy increases the risk of lymphoma over the background risk associated with rheumatoid arthritis.
- Lymphatic Diseases
- Rheumatology Clinical Trials
- Rheumatoid Arthritis
- Leukemia
Kimme L. Hyrich, MD, The University of Manchester, Manchester, United Kingdom, presented data from the British Society for Rheumatology Biologics Register, a large cohort study showing no evidence that antibody to tumor necrosis factor (anti-TNF) therapy increases the risk of lymphoma over the background risk associated with rheumatoid arthritis (RA).
The introduction of anti-TNF therapy almost 20 years ago led to a fundamental shift in the treatment paradigm for RA. However, in 2002 concerns began to appear regarding the possibility of an association between anti-TNF therapy and an increased risk of lymphoma in this patient population [Brown SL et al. Arthritis Rheum 2002]. Assessing this risk is difficult because individuals with RA already have a 2 to 3 times higher risk of lymphoma compared with the general population, and this risk increases with increasing disease severity [Baecklund E et al. Arthritis Rheum 2006]. To date, neither clinical trials [Leombruno JP et al. Ann Rheum Dis 2009] nor observational studies [Setoguchi S et al. Arthritis Rheum 2006; Wolfe F and Michaud K. Arthritis Rheum 2007; Askling J et al. Ann Rheum Dis 2009] have shown such a relationship.
The purpose of this prospective cohort study was to determine whether the use of anti-TNF therapy influences the risk of lymphoma. The study population comprised patients with RA but without prior lymphoproliferative malignancy who were being treated in routine clinical practice in the United Kingdom. Cohort 1 included patients newly exposed to anti-TNF therapy. Cohort 2 included biologic-naïve patients starting or changing to a disease-modifying antirheumatic drug (DMARD). Patient characteristics are shown in Table 1. All participants were followed with both physician and patient questionnaires and linked with the National Health Service cancer and death registry for lymphoma or death. The current results represent follow-up through September 30, 2010. The primary study outcome was risk of first lymphoma in patients ever exposed to anti-TNF therapy versus those exposed to nonbiologic DMARD only. The secondary outcome was the risk of non-Hodgkin lymphoma only.
Baseline Characteristics.
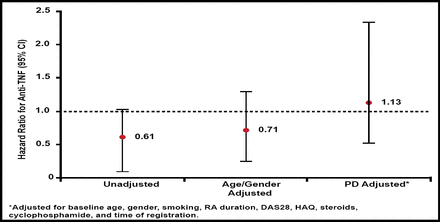
There was no increased risk for lymphoma with anti-TNF treatment compared with nonbiologic DMARD only. The adjusted HR for anti-TNF treatment was 1.13 (Figure 1). In the DMARD group 20% had Hodgkin lymphoma versus 14% in the anti-TNF group. A very similar pattern of risk was noted when limited to non-Hodgkin lymphoma (HR=1.26).
Hazard for Lymphoma (nbDMARD Referent).
DAS=Disease Activity Score; HAQ=Health Assessment Questionnaire; nbDMARD=nonbiologic disease-modifying antirheumatic drug; RA=rheumatoid arthritis; TNF=tumor necrosis factor.
Reproduced with permission from KL Hyrich, MD.
The strengths of the study include it being a large, national cohort with detailed patient data from the National Health Service registry and a propensity model that allowed for adjustment of a large number of covariates. It was limited by a reporting lag, possible screening bias, and the fact that it did not include data on changes in disease activity over time. Further follow-up is recommended to allow for longer latency.
- © 2012 MD Conference Express®