Summary
With evidence accumulating that the β-cells of patients with type 1 diabetes (T1DM) can be infected with enterovirus [Richardson SJ et al. Diabetologia 2009], the question if enterovirus could cause β-cell damage and T1DM has become increasingly relevant [Tauriainen S et al. Semin Immunopathol 2011]. This article discusses the evidence of viral infection in the pancreas of patients with T1DM.
- Viral Infections
- Vaccinations
- Diabetes Mellitus
With evidence accumulating that the β-cells of patients with type 1 diabetes (T1DM) can be infected with enterovirus [Richardson SJ et al. Diabetologia 2009], the question if enterovirus could cause β-cell damage and T1DM has become increasingly relevant [Tauriainen S et al. Semin Immunopathol 2011]. Noel G. Morgan, PhD, University of Exeter, Exeter, United Kingdom, discussed the evidence of viral infection in the pancreas of patients with T1DM.
A systematic review and meta-analysis recently found a clinically significant association between enterovirus infection detected with molecular methods and autoimmunity/T1DM [Yeung WC et al. BMJ 2011]. While observational studies cannot assign causation, the results provide additional support to direct evidence of enterovirus infection in pancreatic tissues of individuals with T1DM [Dotta F et al. Proc Natl Acad Sci USA 2007].
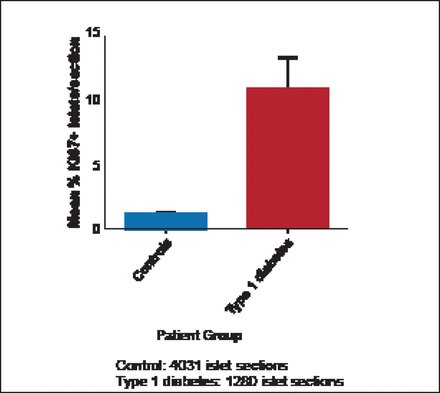
Willcox et al. [Diabetologia 2010] found that α-and β-cells undergo a marked increase in proliferation during the progression of T1DM in humans (Figure 1). These results imply that islet cell proliferation is re-initiated in response to the autoimmune attack associated with T1DM.
Islet Cell Proliferation Is Increased in Response to Insulitis.
Reprinted from Willcox A et al. Evidence of increased islet cell proliferation in patients with recent-onset type 1 diabetes. Diabetologia 2010;3(9):2020–2028, with permission from Springer Verlag.
In 2007, Dotta et al. [Proc Natl Acad Sci USA 2007] found direct evidence that the enterovirus can infect β-cells in patients with T1DM, and infection is associated with inflammation and functional impairment. Two years later, Richardson et al. [Diabetologia 2009] demonstrated that the immunoreactive enteroviral capsid protein vp1 is commonly found in the islets of recent-onset diabetes patients but only rarely in normal pediatric controls. A strong correlation was also seen between islet cell vp1 positivity and protein kinase R (PKR) production in insulin-containing islets of type 1 and type 2 diabetic patients.
The antiapoptotic protein Mcl-1 is also expressed in β-cells [Richardson SJ et al. In press]. PKR activation leads to degradation of Mcl-1, thereby enhancing the sensitivity of cells to proapoptotic stimuli. In the β-cells of patients with T1DM, double-stranded ribonucleic acid (dsRNA), which forms during replication of enteroviruses, has also been detected and appears to be associated with elevated levels of MDA5 (a cellular sensor for dsRNA). Prof. Morgan said, “This is consistent with the possibility that β-cells can sustain an enteroviral infection in patients with T1DM.”
The Possible Roles of Viral Infections and Diabetes
Matthias von Herrath, MD, La Jolla Institute for Allergy and Immunology, San Diego, California, USA, discussed whether viral infection enhances or abrogates T1DM.
Viral infections have been implicated in the etiology of T1DM for more than 100 years [Knip M, Simell O. Cold Spring Harb Perspect Med 2012]. Recent data from Ylipaasto et al. [Diabetologia 2012] suggest that a distinct, virus-strain-specific, gene expression pattern leads to pancreatic islet destruction and proinflammatory effects after enterovirus infection. However, neither viral replication nor cytotoxic cytokine production alone are sufficient to induce necrotic cell death. More likely, the combined effect of these, and possibly cellular energy depletion, is behind the enterovirus-induced necrosis of islets.
Dr. Herrath reported that viral infections cause strong inflammation and/or upregulation of major histocompatibility complex (MHC) molecule 1 and interferons. In animal models for virally induced T1DM, MHC class 1 upregulation of β-cells is a prerequisite for their recognition and demise by CD8+ (killer) T cells [Seewaldt S et al. Diabetes 2000]. Dr. Herrath said that even without an inflammatory infiltrate, human islets can express MHC 1—often many years after diagnosis.
Upregulation of MHC 1 is not uniform; it is patchy, almost in a vitiligo-like fashion. Thus, infections might act as providers of a “fertile field” to facilitate destruction of β-cells by autoreactive CD8s that are detected in human islets.
Conversely, viruses might prevent T1DM through various mechanisms. These are usually operational to curb immunopathology following infections. These mechanisms comprise apoptosis of antiviral and autoreactive (as bystanders) lymphocytes via programmed death ligand-1 and tumor necrosis factor-β mechanisms as well as enhancement of regulatory T cells function and expansion.
Toll-like receptor (TLR) 2 and TLR9 are involved but TLR4 and 7 are not. Viruses that replicate to high levels in their hosts are more likely to accelerate T1DM, whereas lower-level infections are more likely to stop T1DM. Therefore, development of vaccinations against entero- and rota-viruses might help prevent some T1DM in children at risk.
Prospects for a Preventive Enterovirus Vaccine for T1DM
Heikki Hyöty, MD, PhD, University of Tampere, Tampere, Finland, discussed the evidence and prospects for a preventive vaccine against T1DM.
Viruses cause diabetes in animals, including the Coxsackie B and encephalomyocarditis viruses in mice, the Ljungan virus in voles, the Kilham virus in rats, and bovine viral diarrhea in cattle. In humans, enteroviruses have tropism to pancreatic islets.
Enterovirus proteins and RNA are both found in islets but much less so in the exocrine pancreas. Fatal Coxsackie B virus infections cause severe islet cell damage. Coxsackievirus and adenovirus receptor (CAR) is the major receptor for Coxsackie B viruses and is strongly expressed in the islets. Moreover, islet cells are permissive for enteroviruses in vitro.
Ylipaasto et al. [Diabetologia 2004] found a definite islet-cell tropism of enteroviruses in the human pancreas. Oikarinen et al. [Diabetologia 2008] determined that, in children, positivity of islet cell antibodies alone, even when lasting for more than a year, was not associated with inflammatory changes in the islets. However, it was likely the pancreatic islets were infected by an enterovirus.
Epidemiological studies also show an association between enterovirus infections and T1DM. As mentioned previously, Yeung WC et al. [BMJ 2011] identified a clinically significant association between enterovirus infection detected with molecular methods and autoimmunity/T1DM. Changes in the epidemiology of enterovirus infections can explain the increasing incidence of T1DM, variation in diabetes incidence between countries, and seasonal onset of the disease. Prof. Hyöty said that the possibilities to develop a vaccine against enteroviruses should be fully explored. The first such studies are currently in progress. The identification of the exact subtypes of enteroviruses that show association with T1DM is an important goal in these studies. If these subtypes can be identified, the production of the vaccine would become technically possible.
- © 2012 MD Conference Express®