Summary
The burden of hepatitis C (HCV) in the United States now exceeds that of HIV and hepatitis B combined. It is estimated that from 2010 to 2019, 193,000 HCV-related deaths, direct medical care costs of $10.7 billion, and $75.3 billion in societal costs can be expected. HCV-associated liver related morbidity and mortality is an important component of this burden [Wong JB et al. Am J Public Health 2000]. This article discusses how antiviral therapy targeted to achieve viral eradication is the key strategy to stabilizing or reversing liver injury and fibrosis, and reducing the risk of liver-related complications, including cirrhosis and liver cancer, in patients with HCV.
- Viral Infections
The burden of hepatitis C (HCV) in the United States now exceeds that of HIV and hepatitis B combined. It is estimated that from 2010 to 2019, 193,000 HCV-related deaths, direct medical care costs of $10.7 billion, and $75.3 billion in societal costs can be expected. HCV-associated liver related morbidity and mortality is an important component of this burden [Wong JB et al. Am J Public Health 2000]. Norah Terrault, MD, University of California, San Francisco, USA, discussed how antiviral therapy targeted to achieve viral eradication is the key strategy to stabilizing or reversing liver injury and fibrosis, and reducing the risk of liver-related complications, including cirrhosis and liver cancer, in patients with HCV.
Patients with genotype 1 were previously considered to be the poorest responders to treatment, but a recent study has shown significant increases in sustained virologic response (SVR) when these patients are treated with peginterferon plus ribavirin (PegIFN+RBV) plus a protease inhibitor (PI; boceprevir or telaprevir), whether they are treatment naïve [Poordad F et al. N Engl J Med 2011; Jacobson IM et al. N Engl J Med 2011] or have been previously treated [Bacon BR et al. N Engl J Med 2011; McHutchison JG et al. New Engl J Med 2010]. However, despite these overall impressive improvements in SVR rates with the PI combinations, there are still difficult-to-cure patient populations such as African Americans, patients with cirrhosis, and partial and null responders to prior PegIFN + RBV therapy. The other issue of concern is treatment-emergent resistance, which is associated with prior nonresponse or poor response to PegIFN+RBV, subtype (1a is more resistant than 1b), absence of ribavirin, and initial high viral loads. However, over time resistance variants disappear and are replaced by wild-type virus in about 0.8 to 10.0 months [Sullivan JC. EASL 2011].
New drugs for HCV continue to be identified and tested; the field of HCV therapy is expected to significantly change in the years to come. Triple therapy with response-guided duration is the new standard of care. The future will bring new triple and quad therapies, and the possibility of IFN-free regimens. New classes of drugs that include more potent second generation PIs, polymerase inhibitors, and NS5A inhibitors as well as host-targeted agents, such as cyclophilin inhibitors, are being tested.
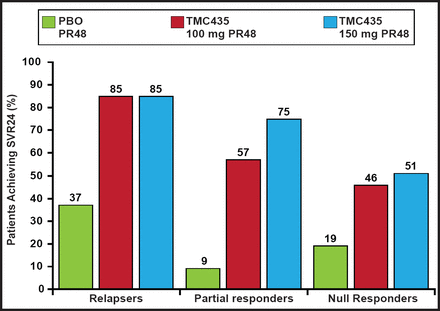
In the ASPIRE study a PI, TMC435, added to PegIFN+RBV resulted in higher SVR rates in relapsers, partial responders, and null responders compared with placebo+RBV treated patients (Figure 1) [Zeuzem S. EASL 2012]. NS5A inhibitors offer potent antiviral activity, an average side effect profile, variable genotype coverage, and a low-to-average barrier to resistance. Daclatasvir+RBV produced significantly higher SVR rates at all doses compared with placebo+RBV in treatment-naïve genotype 1 patients. With nucleoside polymerase inhibitors+RBV, which has demonstrated a high barrier to resistance and pangenotypic activity in treatment-naïve genotype 1 patients, achieved SVR rates as high as 92%. Quad therapy may reduce resistance risk, improve potency, offer shorter duration of treatment, and offer advantages in difficult-to-cure populations.
ASPIRE Study: TMC435 plus RBV in Genotype Treatment-Experienced.
RBV=ribavirin; SVR=sustained virologic response; PBO=placebo.
Dr. Terrault noted that although SVR rates have improved with the use of PI-triple therapy, there is still a need for better drug therapies, especially in difficult-to-cure populations, in which SVR rates are still ≤50%. Additionally, current PI-triple therapy has limited genotype coverage, requires long (48-week) treatment duration in some patients, is associated with frequent side effects, has drug-drug interactions, and has resistance issues. The next generation of triple or quad therapies is expected to have higher SVR rates, a shorter duration of therapy, broader genotype application, and simpler regimens. Deciding whether to treat now or wait for future therapies depends on the likelihood of response and risk of waiting, tolerability of PegIFN + RBV, and practical issues such as insurance status and home/work support.
- © 2012 MD Conference Express®