Summary
Children infected with Mycobacterium tuberculosis have a significant risk of developing tuberculosis (TB) and therefore can benefit from preventive therapy and new diagnostic techniques. This article discusses the advantages and disadvantages of some new diagnostic techniques being used to test children for TB.
- Vaccinations
- Bacterial Infections
Children infected with Mycobacterium tuberculosis have a significant risk of developing tuberculosis (TB) and therefore can benefit from preventive therapy and new diagnostic techniques. Jeffrey R. Starke, MD, Baylor College of Medicine, Houston, Texas, USA, discussed the advantages and disadvantages of some new diagnostic techniques being used to test children for TB.
The standard method for diagnosing TB in children consists of a positive tuberculin skin test (TST), an abnormal chest X-ray and/or physical exam, and a history of recent contact with an infectious adult case of TB. However, new diagnostic techniques are being developed. Those that are successful in adults will need to be tested in children.
The interferon-γ release assay (IGRA) offers several potential advantages over TST and can detect host response to Mycobacterium tuberculosis-specific antigens. Two IGRA assays are currently available. Study results have shown that the IGRA and TST have similar accuracy for detection of TB infection and the diagnosis of disease in children. Compared with TST, a higher specificity with IGRA performance has been suggested, making these tests particularly advantegous in children receiving bacillus Calmette–Guérin (BCG) vaccination [Mandalakas AM et al. Int J Tuberc Lung Dis 2011; Chiappini E et al. Int J Immunopathol Pharmacol Dis 2012].
Under current pediatric guidelines the TST is preferred when testing for TB infection in children aged <5 years. In children aged ≥5 years who have received BCG vaccine or who are unlikely to return for TST reading, the IGRA is preferred. Both tests should be considered when the initial and repeat IGRA results are indeterminate, when either test is negative and there exists clinical suspicion for TB disease; when the risk of infection with poor outcome is higher; when the initial TST is positive and the child is aged ≥5 years and has a history of BCG vaccination; when there is a need for additional evidence to increase compliance; and if nontuberculous mycobacteria disease is suspected.
Giuseppe Indolfi, MD, PhD, Meyer Children's Hospital, Florence, Italy, reviewed the current hepatitis treatment options in children. One of the characteristics of hepatitis B (HBV) in children is that it is often a chronic infection, particularly when contracted by a newborn. The optimal treatment for chronic HBV infection in children should ideally be started as early as possible before the immune-active phase (when the liver first starts accumulating inflammation and fibrosis) and should be directed toward eradicating HBV and obtaining HBV surface antigen loss [Indolfi G. Fut Virol in press]. FDA approved drugs for children include lamivudine (≥3 years), tenofovir and adefovir (≥12 years), entecavir (≥16 years), and interferon (≥1 year). Guidelines and consensus of expert panels recommend a conservative treatment approach. When needed, the first therapeutic option is treatment of finite duration with interferon to obtain sustained off-therapy virological response. The use of long-term nucleotide analogues can be aimed at obtaining sustained off-therapy virological response but can also result in long-term on-therapy virological response. Children in the immune-active phase for >3 months should be considered for therapy, while there is no indication for treatment in the immune-tolerant phase outside the context of clinical trials.
Hepatitis C virus (HCV) is primarily transmitted perinatally. Chronic infection in children can be asymptomatic and spontaneous clearance is possible. Overall, HCV is a mild disease in children. Cirrhosis is rare (<2%). In general, the chance of liver fibrosis seems to increase either with patient age, disease duration, or both. Although data are discordant, older adolescents and young adults tend to have more severe fibrosis than children. Peginterferon α-2b (1.5 μg/kg per week), peginterferon α-2a (100 μg/m2 per week, max 180 μg), and ribavirin (15 mg/kg per day, max 1200 mg) have been approved for treatment of HCV. Treatment duration is 48 weeks for genotypes 1, 4, 5, and 6, and 24 weeks for genotypes 2 and 3. Combination therapy is more effective and better tolerated in children when compared with adults. Sustained virological response in children with genotype 1 infection is around 55%, but it is higher (>90%) in children infected by HCV genotypes 2 and 3. The latter result strongly supports the treatment of children with HCV genotype 2 and 3 infection. A conservative approach is suggested for children infected with HCV genotype 1, given the mild natural history of the disease in childhood and the new highly effective treatment options available for adults.
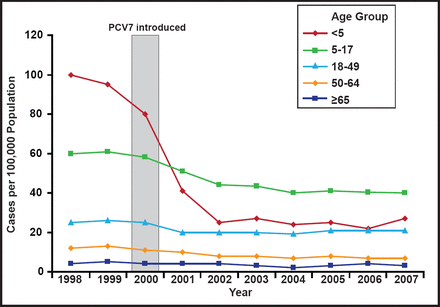
When it was introduced in 2000, pneumococcal conjugate vaccine (PVC7) contained serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F, which accounted for 80% of invasive disease in children aged <5 years and 89% of penicillin-resistant isolates. By 2006, 93% of children in the United States were receiving 3 doses of PCV7 before the age of 24 months, and by 2007 it was available in more than 70 countries. Following the vaccine's introduction, the incidence of pneumococcal disease (IPD) declined by 45% in all age groups and by 76% in US children aged <5 years (Figure 1) [Pilishvili T et al. J Infect Dis 2010]. There was also a reduction in meningitis and bacteremia. However the proportion of invasive pneumococcal infections caused by serotype 19A increased in children [Kaplan SL et al. Pediatrics 2010]. Possible mechanisms for the increase in 19A serotypes include vaccine-induced serotype replacement and antibiotic pressure [Reinert R et al. Vaccine 2010; Dagan R et al. J Infect Dis 2009; Moore MR. J Infect Dis 2009].
Invasive Pneumococcal Disease in the USA, 1998 to 2007.
Reprinted with permission from the American Society for Microbiology. Pilishvili T et al. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis. 2010;201(1):32–41.
PCV13, which adds serotypes 1, 3, 5, 6A, 7F, and 19A, was licensed in the USA for children in 2010. It is recommended for all children aged 2 to 59 months and high-risk children aged 60 to 71 months. Recently it was also licensed for use in adults aged ≥50 years; however, the Advisory Committee for Immunization Practices has not made recommendations for its use in adults. Several studies have shown significant reductions in the incidence of pneumococcal disease among children in the USA since the introduction of the PCV13 vaccine [Yildirim I et al., Hsu K et al., Lee GM et al. IDSA 2011; Kaplan SL et al. Pediatr Infect Dis J. In press]. The results have been particularly robust among Alaskan Native children who had more than 10 times higher incidence of pneumococcal disease compared with other US children prior to PCV7's introduction [Singleton R et al. ESPID 2011; Bruce M et al. IDSA 2011]. It is estimated that over a 10-year period, PCVC13 will prevent 106,000 cases of IPD and 2.9 million cases of pneumonia, and save $11.6 billion [Rubin JL et al. Vaccine 2010].
The editors would like to thank the many members of the ICAAC Congress 2012 presenting faculty who generously gave their time to ensure the accuracy and quality of the articles in this publication.
- © 2012 MD Conference Express®