Summary
Patients with atrial fibrillation (AF) have a 5-fold risk of stroke and suffer more severe strokes compared with the general population [Camm AJ et al. Eur Heart J 2010]. One-year mortality in stroke patients with AF is 63% versus 34% in those without AF (p<0.001) [Lin H-J et al. Stroke 1996]. The risk of stroke recurrence is slightly higher in patients with AF (6.9% vs 4.7% in those without AF; p=0.0398) [Marini C et al. Stroke 2005].
- Cerebrovascular Disease
- Arrhythmias
Patients with atrial fibrillation (AF) have a 5-fold risk of stroke and suffer more severe strokes compared with the general population [Camm AJ et al. Eur Heart J 2010]. One-year mortality in stroke patients with AF is 63% versus 34% in those without AF (p<0.001) [Lin H-J et al. Stroke 1996]. The risk of stroke recurrence is slightly higher in patients with AF (6.9% vs 4.7% in those without AF; p=0.0398) [Marini C et al. Stroke 2005].
Is There a Role for Vitamin K Antagonists?
A meta-analysis of 5 randomized placebo-controlled trials found that the relative risk reduction with warfarin was 62%, but the incidence of cerebral hemorrhage was substantial in elderly patients taking warfarin [Hart RG et al. Ann Intern Med 1999]. Jean-Yves Le Heuzey, MD, Georges Pompidou Hospital, Rene Descartes University, Paris, France, discussed the challenges of vitamin K antagonists (VKAs), including a narrow therapeutic window, complex kinetics, and multiple interactions. An estimated 50% of eligible patients receive no anticoagulation therapy because of the limitations of VKAs.
Oral anticoagulant (OAC) therapy with an adjusted-dose VKA, a direct thrombin inhibitor, or oral factor Xa inhibitor (is now recommended for patients with CHA2DS2-VASc score ≥1, with preference for a new NOAC over a VKA for most patients with nonvalvular AF [Class I, Level of Evidence A; Camm AJ et al. Eur Heart J 2012].
VKAs still have a role in anticoagulation therapy for patients with AF. The main contraindications for the new anticoagulants are valvular AF, renal failure, and stable INRs in patients unwilling to switch.
Are Trials Comparable?
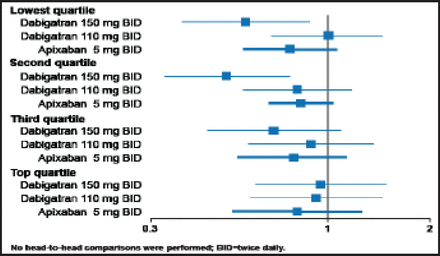
Lars Wallentin, MD, Uppsala University, Uppsala, Sweden, reviewed the available efficacy and safety data for dabigatran, rivaroxaban, apixaban, and edoxaban in patients with AF (Table 1). Analysis by quartiles of time in therapeutic range (TTR) showed that dabigatran and apixaban remained effective for stroke or systemic embolism (SE) reduction irrespective of centers' quality of INR control (Figure 1).
OAC Study Results.
New OACs Compared with Warfarin, Stroke, or SE in Relation to Quartiles of Center's TTR.
Reproduced with permission from The Lancet; Wallentin L et al. Efficacy and safety of dabigatran compared with warfarin at different levels of international normalised ratio control for stroke prevention in atrial fibrillation: An analysis of the RE-LY trial. 2010;376(9745):975–83.
Prof. Wallentin concluded that compared with warfarin, new NOACs provide a better antithrombotic effect, lower risk of bleeding, fewer unexpected side effects, oral bioavailability, fewer food or drug interactions, broad therapeutic window, predictable anticoagulation without laboratory monitoring, and better patient acceptance and long-term tolerance.
Bleeding Risk with Anticoagulant Drugs
Michael D. Ezekowitz, MD, Thomas Jefferson Medical College, Philadelphia, Pennsylvania, USA, discussed the bleeding risks with OACs in AF patients. According to Dr. Ezekowitz, the risk of intracranial bleeding can be reduced by following the inclusion and exclusion criteria used in the clinical trials, assessing renal function, implementing dose adjustment per the prescribing information, and following the presurgical and the missed-dose protocols. He emphasized that none of the trials were stopped early because of increased bleeding or adverse events.
Table 2 shows the annual bleeding event rates in the RE-LY, ROCKET-AF, and ARISTOTLE trials. Compared with warfarin, major bleeding rates were significantly lower with dabigatran 110 mg and also lower with apixaban. Major bleeding rates with dabigatran 150 mg and with rivaroxaban were noninferior to that observed with warfarin. Major bleeding and intracranial hemorrhage (ICH) with warfarin and dabigatran did not correlate with the center-based TTR, dose or anticoagulant, CHADS2, or CHA2DS2-VASc scores.
Bleeding Event Rates.
Analysis of the RE-LY trial efficacy results showed that the stroke and SE rate reduction with dabigatran was independent of CHADS2 score. However, lower major bleeding rates with dabigatran 150 mg versus warfarin were only observed in patients with CHADS2 score 0 to 1, while the benefit with dabigatran 110 mg remained across all CHADS2 scores.
Dr. Ezekowitz concluded that physicians are more influenced by OAC-induced bleeding than by OAC benefits in stroke prevention. The decision to use warfarin for nonvalvular AF is primarily driven by perceived risks of ICH. Fortunately NOACs reduce ICH by approximately 50%.
Impact of Renal Function on Antithrombotic Therapy
Limited data are available on stroke prevention in AF patients with renal dysfunction. Current stroke risk stratification strategies are based on data excluding patients with severe renal dysfunction. The aim of the study presented by Shinya Goto, MD, Tokai University, Kanagawa, Japan, was to assess the impact of renal dysfunction on stroke thromboprophylaxis in AF patients.
Study data were obtained from Cohort 1 of the international Global Anticoagulant Registry in the FIELD (GARFIELD) Registry [Kakkar AK et al. Am Heart J 2012] which included 55,000 patients stratified into prospective (AF diagnosis ≤6 weeks prior) and retrospective (diagnosis 6 to 24 months prior) groups with ≥1 additional stroke risk factors. Data for both groups were combined. Antithrombotic therapy (n=10,537) was analyzed according to kidney function stage.
Glomerular filtration rate data were available for 72% of patients; 67% had Stage 1 kidney dysfunction. VKAs were taken by 57.4% of patients, with 48.2% receiving a VKA alone, 11.7% receiving both a VKA and an antiplatelet, and 26.7% receiving an antiplatelet only. The use of combination therapy increased as the level of kidney dysfunction increased.
In this registry, AF patients with renal dysfunction were more likely to receive combination therapy with antiplatelet and VKA agents than VKA monotherapy.
ROCKET-AF Trial
Manesh R. Patel, MD, Duke Clinical Research Institute, Durham, North Carolina, USA, presented results of the ROCKET-AF trial related to inadequate anticoagulation during end-of-study transition to open-label VKA therapy and the relationship between center TTR (cTTR) and comparative efficacy of rivaroxaban and warfarin.
The ROCKET-AF trial randomized about 14,000 patients to rivaroxaban 20 mg BID or warfarin (INR target 2.0–3.0). At the end of the blinded phase, study therapy was discontinued during transition to open-label VKA therapy. To maintain blinding, local unblinded INR measurements were discouraged for at least 3 days after the start of open-label VKA therapy.
This analysis focused on events 3 to 30 days after the end-of-study visit in patients still on the study drug (n=9248; 65%). Of these patients, 92% were transitioned to open-label VKA within 30 days. The median time to therapeutic INR after end of study in these patients was 3 days in those assigned warfarin and 13 days in those assigned rivaroxaban. From Days 3 to 30, the stroke and SE rate/100 patient-years was 6.42% with rivaroxaban versus 1.73% with warfarin (HR, 3.72; 95% CI, 1.51 to 9.16; p=0.004) [Johnson & Johnson. Advisory Committee Briefing Document, No. EDMS-ERI-24510755:2.0 2011]. The excess events with rivaroxaban likely were caused by inadequate rates of and delayed achievement of therapeutic warfarin levels during this time.
A second analysis explored the impact of the quality of warfarin therapy by analyzing cTTR and treatment effect. The median TTR (INR 2.0–3.0) among warfarin patients was 57.8%. The treatment effect with rivaroxaban on stroke plus SE was consistent across cTTR quartiles (p for interaction=0.73). Estimated HRs for rivaroxaban versus warfarin favored rivaroxaban over a wide range of cTTRs.
- © 2012 MD Conference Express®