Summary
Treatment with a low dose of rivaroxaban, an oral factor Xa inhibitor, has been shown to reduce recurrent cardiovascular (CV) events and offer a survival benefit without a significant increase in fatal bleeding for patients who have had an ST-segment elevation myocardial infarction. Rivaroxaban reduced recurrent CV events across the spectrum of acute coronary syndrome in the Anti-Xa Therapy to Lower Cardiovascular Events in Addition to Standard Therapy in Subjects with Acute Coronary Syndrome—Thrombolysis in Myocardial Infarction 51 [ATLAS ACS 2-TIMI 51; J Mega et al. N Engl J Med 2012] trial.
- Cardiology Clinical Trials
- Myocardial Infarction
- Thrombotic Disorders
- Featured Meeting - Specialty page
- Coronary Artery Disease
Treatment with a low dose of rivaroxaban, an oral factor Xa inhibitor, has been shown to reduce recurrent cardiovascular (CV) events and offer a survival benefit without a significant increase in fatal bleeding for patients who have had an ST-segment elevation myocardial infarction (STEMI).
Rivaroxaban reduced recurrent CV events across the spectrum of acute coronary syndrome in the Anti-Xa Therapy to Lower Cardiovascular Events in Addition to Standard Therapy in Subjects with Acute Coronary Syndrome– Thrombolysis in Myocardial Infarction 51 [ATLAS ACS 2-TIMI 51; J Mega et al. N Engl J Med 2012] trial. Jessica L. Mega, MD, MPH, Brigham and Women's Hospital, Boston, Massachusetts, USA, reported the findings from an analysis of the prespecified subgroup in 7187 patients with STEMI in that trial. Effective long-term anticoagulant therapy has been of particular interest in this subpopulation because of prior studies that demonstrated an increase in thrombin production that lasted several months after STEMI.
The patients were randomly assigned to treatment with rivaroxaban at 2.5 mg BID (n=2601) or 5 mg BID (n=2584) or to placebo (n=2632). The patients received thienopyridine at the physician's discretion, as well as aspirin at a dose of 75 to 100 mg QD. The primary efficacy endpoint was a composite of CV death, MI, or stroke, and the primary safety endpoint was TIMI major bleeding not associated with coronary artery bypass grafting.
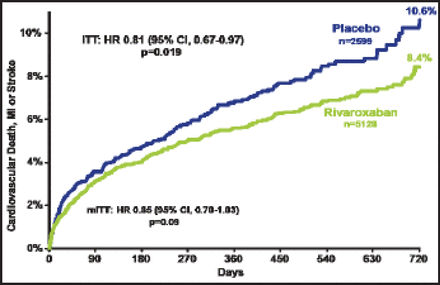
The primary efficacy endpoint occurred in significantly fewer patients in both rivaroxaban groups (Figure 1). The benefit of rivaroxaban was apparent as early as 30 days—1.7% in the combined rivaroxaban groups compared with 2.3% in the placebo group (HR, 0.71; 95% CI, 0.51 to 0.99; p=0.042 for the intention to treat group). When each rivaroxaban group was compared with placebo, both were associated with a lower rate of the primary efficacy endpoint: 10.6% for placebo versus 8.7% for the 2.5 mg group (p=0.047) versus 8.2% for the 5 mg group (p=0.051). However, only the lower dose was associated with a significantly lower rate of CV death: 4.2% for placebo versus 2.5% for the 2.5 mg group (p=0.006) versus 4.0% for the 5 mg group (p=0.64).
Cardiovascular Death, MI, or Stroke.
ITT=intention to treat; MI=myocardial infarction; mITT=modified ITT.
Reproduced with permission from J. Mega, MD, MPH.
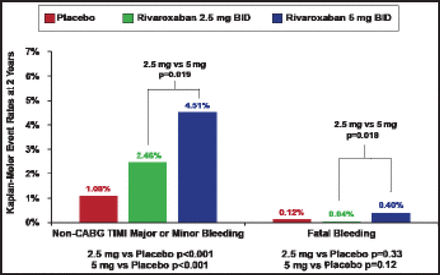
The primary safety endpoint was significantly increased in both rivaroxaban groups, with rates of 1.7% (2.5 mg group), 2.7% (5 mg group), and 0.6% (placebo; p<0.001 for comparison of either dose with placebo; Figure 2). However, fatal bleeding was not significantly increased with rivaroxaban: 0.12% (placebo) versus 0.04% (2.5 mg rivaroxaban; p=0.33) versus 0.40% (5 mg rivaroxaban; p=0.12).
Other Safety Endpoints.
CABG=coronary artery bypass graft; TIMI=thrombolysis in myocardial infarction.
Reproduced with permission from J. Mega, MD, MPH.
Dr. Mega and colleagues concluded that treatment with rivaroxaban 2.5 mg BID offers an effective strategy to reduce thrombotic events in patients following STEMI.
- © 2012 MD Conference Express®