Summary
Results of the first randomized trial to address optimal antiplatelet therapy in patients on oral anticoagulants (OACs) undergoing coronary stenting showed that treatment with dual antithrombotic therapy (OAC plus clopidogrel alone) caused less bleeding than triple antithrombotic therapy (OAC plus aspirin plus clopidogrel), with no excess of thrombotic/thromboembolic events and less all-cause mortality. This article discusses the What Is the Optimal Antiplatelet and Anticoagulant Therapy in Patients with Oral Anticoagulation and Coronary Stenting [WOEST; NCT00769938] trial.
- Interventional Techniques & Devices
- Cardiology Clinical Trials
- Arrhythmias
- Thrombotic Disorders
Results of the first randomized trial to address optimal antiplatelet therapy in patients on oral anticoagulants (OACs) undergoing coronary stenting showed that treatment with dual antithrombotic therapy (OAC plus clopidogrel alone) caused less bleeding than triple antithrombotic therapy (OAC plus aspirin plus clopidogrel), with no excess of thrombotic/thromboembolic events and less all-cause mortality.
Long-term OAC therapy is necessary in most patients with atrial fibrillation (AF) or a mechanical heart valve. The addition of aspirin and clopidogrel are indicated when these patients undergo percutaneous coronary intervention (PCI), but when all 3 drugs are coadministered, the risk of major bleeding is substantially increased [Sorensen et al. Lancet 2009]. In this context, the What Is the Optimal Antiplatelet and Anticoagulant Therapy in Patients with Oral Anticoagulation and Coronary Stenting [WOEST; NCT00769938] trial was designed to test the hypothesis that in patients on OAC therapy undergoing PCI, the addition of clopidogrel alone is superior to the combination of aspirin and clopidogrel with respect to bleeding. The results of the trial were presented by Willem Dewilde, MD, Twee Steden Hospital, Tilburg, the Netherlands.
WOEST was an investigator-initiated, prospective, randomized study conducted in 15 Danish and Belgium hospitals between November 2008 and November 2011. Patients with a prior history of AF (69%), a mechanical heart valve (10%), or other indication for OAC (eg, thromboembolic disease or severe systolic heart failure) were openly randomized to either dual (warfarin+clopidogrel 75 mg QD; n=279) or triple (warfarin+clopidogrel 75 mg QD+aspirin 80 mg QD; n=284) therapy. Patients were treated with clopidogrel for a minimum of 1 month after placement of a bare-metal stent (∼30% of patients) and 1 year after placement of a drug-eluting stent (∼65%). All patients were followed for 1 year. The primary outcome was the occurrence of any bleeding event (Thrombolysis in Myocardial Infarction [TIMI] major or minor criteria), and the study was statistically powered to detect a 60% reduction in bleeding based on prior cohort data (annual expected bleeding rate on triple therapy was projected to be 12%). Secondary exploratory endpoints included ischemic events, a combination of stroke, death, myocardial infarction (MI); stent thrombosis (ST) and target vessel revascularization (TVR); and individual components of these endpoints [Dewilde W and Berg JT. Am Heart J 2009].
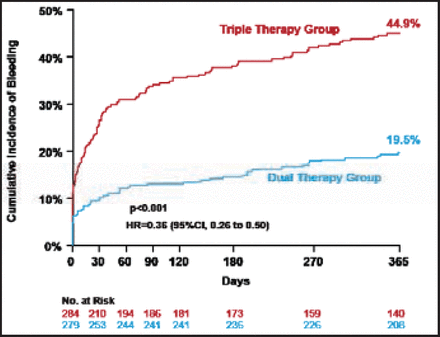
Baseline characteristics in the WOEST study revealed the mean age of patients was ∼70 years, ∼80% were men, ∼70% had a history of hypertension, 70% had hypercholesterolemia, and 25% to 30% had a prior history of either diabetes, MI, or heart failure. Concurrent use of proton pump inhibitors was ∼35%. Despite the exclusion of patients with a recent history of major bleeding, peptic ulcer disease, or other major risk factors, bleeding in this study was higher than expected (∼45% of patients assigned to triple therapy experienced a bleeding endpoint within 1 year). However, patients treated with dual therapy experienced significantly less bleeding compared with triple therapy (19.5% vs 44.9%; HR, 0.36; 95% CI, 0.26 to 0.50; p<0.001; Figure 1).
Primary Endpoint: Total Number of Bleeding Events (TIMI Criteria).
TIMI=thrombolysis in myocardial infarction.
Reproduced with permission from W. Dewilde, MD.
Results were consistent among major subgroups, including when analyzed by a threshold age of 75 years, gender, presentation with acute coronary syndrome, indication for OAC, and stent received. The difference in bleeding between the 2 treatment groups was driven predominantly by TIMI minimal and minor bleeding from the access site, gastrointestinal, and superficial locations. There was no difference between the 2 groups in TIMI major bleeding (3.3% vs 5.8%; p=0.159) or intracranial bleeds (3 in each group).
Patients receiving dual therapy experienced significantly fewer composite ischemic events compared with those receiving triple therapy (11.3% vs 17.7%; HR, 0.60; 95% CI, 0.38 to 0.94; p=0.025). Each component of the composite ischemic endpoint was consistently less frequent among patients assigned dual therapy except for TVR.
The investigators concluded that a strategy of omitting aspirin is an option in high-risk patients on chronic anticoagulation undergoing PCI. Although it was an open-label study, this provocative trial will hopefully open the door for further investigations of the optimal long-term treatment strategy to balance ischemic and bleeding outcomes in high-risk patients.
- © 2012 MD Conference Express®