Summary
Abdominal obesity is a marker of dysfunctional adipose tissue [Després JP, Lemieux I. Nature 2006]. This article provides an overview of alterations in visceral and subcutaneous adipose tissue function in individuals with visceral obesity, with an emphasis on adipose tissue metabolism. He also discussed fat cell size, adipocyte hypertrophy and storage capacity; adipose tissue lipolysis; excess substrate and inflammation; mesenteric adipose tissue; and abdominal fat in severe obesity.
- Cardiometabolic Disorder
- Obesity
Abdominal obesity is a marker of dysfunctional adipose tissue [Després JP, Lemieux I. Nature 2006]. André Tchernof, PhD, Université Laval, Québec City, Québec, Canada, provided an overview of alterations in visceral and subcutaneous adipose tissue function in individuals with visceral obesity, with an emphasis on adipose tissue metabolism. He also discussed fat cell size, adipocyte hypertrophy and storage capacity; adipose tissue lipolysis; excess substrate and inflammation; mesenteric adipose tissue; and abdominal fat in severe obesity.
In a study of regional differences in adipocyte metabolism and visceral versus subcutaneous parameters across adiposity values in women, Tchernof et al. [Diabetes 2006] found that compared with omental adipocytes, subcutaneous adipocytes are larger, have higher lipoprotein lipase activity, and are more lipolytic on an absolute basis—factors that may reflect higher fat storage capacity. They also demonstrated that overall and visceral obesity had only minor effects on regional differences in adipose tissue metabolism.
Veilleux et al. [Diabetes 2011] reported that women characterized by omental adipocyte hypertrophy presented a deleterious lipid profile compared with those characterized by omental hyperplasia. Findings indicate that a 10% enlargement of omental adipocytes increased the risk of hypertriglyceridemia more than 4-fold, whereas enlarged subcutaneous adipocytes failed to significantly alter the risk of hypertriglyceridemia.
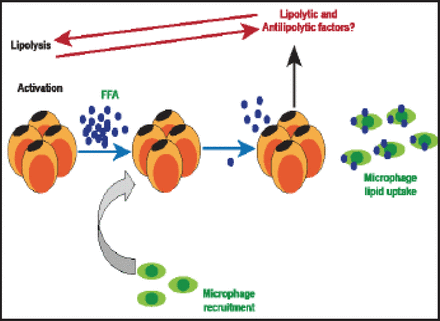
Obesity engenders a complex immune response in which macrophage accumulation in adipose tissue is a characteristic feature [Kosteli A et al. J Clin Invest 2010]. In women, visceral fat accumulation is an indicator of adipose tissue macrophage infiltration, and it is the best correlate of macrophage infiltration in both subcutaneous and fat compartments of lean to obese women [Michaud A et al. Metabolism 2012]. According to Kosteli et al. [J Clin Inves 2010] excess lipolysis could be one of the causal factors for adipose tissue macrophage infiltration (Figure 1).
Lipolysis, Macrophage Infiltration, and Inflammation.
Reproduced with permission from A. Tchernof, PhD.
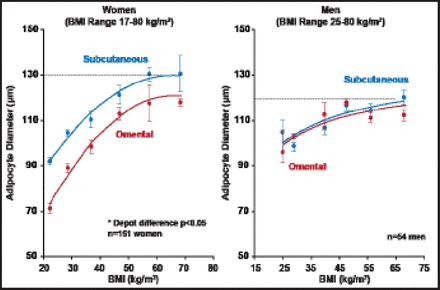
Regarding the correlations of fat cell size with obesity and fat distribution, all adiposity measures, including body mass index (BMI) and total body fat mass, as well as adipose tissue areas measured by computed tomography, are strongly and positively correlated with subcutaneous and omental adipocyte diameter (Figure 2) [Tchernof A et al. Diabetes 2006].
Adipocyte Diameter as a Function of BMI in Men and Women.
Reproduced with permission from A. Tchernof, PhD.
Prof. Tchernof noted that in the lean to moderately obese range, visceral adipocyte hypertrophy is clearly related to dyslipidemia, independent of total adiposity and body fat distribution. However, the fact that fat cell size reaches a plateau in individuals with severe obesity suggests it may not predict metabolic complications in this population. This conclusion coincides with reports from Lemieux et al. [Diabetes Care 2006] and Drapeau et al. [Obes Surg 2007] that cast doubt on the utility of waist circumference to predict metabolic abnormalities and complications in severely obese men and women.
Prof. Tchernof concluded the following:
-
Visceral adipose tissue expands mostly through adipocyte hypertrophy and quickly becomes inefficient in storing excess lipids.
-
Limited adipose tissue lipid storage capacity has emerged as a critical determinant of cardiometabolic alterations.
-
Lipolytic responsiveness of visceral adipocytes to positive stimuli is increased in visceral obesity, but most studies support a reduced inhibitory response to insulin in visceral adipocytes compared with subcutaneous adipocytes.
-
Visceral obesity and high lipolytic rates are closely related to macrophage infiltration and inflammation.
-
The pathophysiology of metabolic disorders in severe obesity may no longer be related solely to excess visceral fat and/or visceral adipocyte hypertrophy.
- © 2012 MD Conference Express®