Summary
This article discusses management of heart failure in the 21st century, including successes, failures, new treatments, and telecardiology.
- Heart Failure
Michel Komajda, MD, Pitié-Salpětrière Hospital, Paris, France, discussed management of heart failure (HF) in the 21st century, including successes, failures, new treatments, and telecardiology.
Successes and Failures
Improvements in HF management can be attributed to the advent of angiotensin-converting enzyme (ACE) inhibitors and beta-blockers. Currently, all major guidelines recommend the combination of ACE inhibitors (or angiotensin II receptor blockers [ARBs]) and beta-blockers for reducing morbidity and mortality in patients with chronic HF and low ejection fraction (EF) levels. The magnitude of success was demonstrated by the reduction in mortality rates from 15.7% in 1991 in SOLVD-T [SOLVD Investigators. N Engl J Med 1991] to 6.1% in 2003 in CHARM-Added [McMurray JJ et al. Lancet 2003] with the use of ACE inhibitors, beta-blockers, and ARBs. The annual incidence of first hospitalization for HF has also decreased. The European Society of Cardiology (ESC) registry for HF (n=3226) reported prescription rates of ACE inhibitors/ARBs and beta-blockers close to 90%, with aldosterone blockers at 43%. There is room for improvement, however, with all-cause mortality rates and all-cause mortality/HF hospitalization rates of 6.8% and 17.2% in chronic HF and 16.8% and 35.1% in acute HF, respectively (ESC registry).
Two recent trials have shown positive results in chronic HF:
-
The SHIFT trial, which studied patients with chronic HF, low EF levels, and increased heart rate, showed that cardiovascular (CV) death or HF hospitalization was significantly reduced by 18% with the selective sinus-node inhibitor ivabradine versus placebo (HR, 0.82; p<0.0001). Hospitalization for worsening HF was reduced by 26% (HR, 0.74; 0.66 to 0.83; p<0.0001) [Swedberg K et al. Lancet 2010]. Other studies of ivabradine demonstrated significantly improved quality of life [Ekman I et al. Eur Heart J 2011] and left ventricular end systolic volume index and left ventricular EF (LVEF) [Tardif JC et al. Eur Heart J 2011].
-
The EMPHASIS trial of eplerenone (n=2737) was stopped prematurely because of the overwhelming benefit of eplerenone versus placebo in reducing CV death or HF hospitalization (18.3% vs 25.9%; HR, 0.63; 95% CI, 0.54 to 0.74; p <0.001) [Zannad F et al. N Engl J Med 2011].
Much has been learned about implantable cardioverter defibrillators (ICDs) and cardiac resynchronization therapy (CRT). The SCD-HeFT trial demonstrated a significant reduction in mortality with ICDs versus placebo (p=0.007) and amiodarone (amiodarone vs placebo; p=0.53) [Bardy GH et al. N Engl J Med 2005]. Two pivotal trials reported a significant benefit with CRT versus medical therapy in event-free survival (EFS; CV death or hospitalization). EFS was improved in the COMPANION trial [Bristow MR et al. N Engl J Med 2004] with CRT-defibrillators (CRT-D; p<0.001) and CRT-pacemakers (CRT-P; p=0.002) and in the CARE-HF study [Cleland JG et al. N Eng J Med 2005], with CRT-P (p<0.001). Failures concern acute or acutely decompensated HF, and HF with preserved EF. Not all acute HF trials have shown positive results. The ESSENTIAL trial of low-dose enoximone [Metra MM et al. Eur Heart J 2009], the SURVIVE and REVIVE trials of levosimendan [Mebazza A et al. JAMA 2009], and the EVEREST trial of tolvaptan [Udelson JE et al. J Am Coll Cardiol 2008] all had negative results. The PROTECT trial found no benefit with the adenosine antagonist rolofylline versus placebo [Voors AA et al. J Am Coll Cardiol 2011]. Likewise, nesiritide was not superior to placebo with respect to 30-day death/HF rehospitalization, 30-day death, or HF rehospitalization (p=0.31).
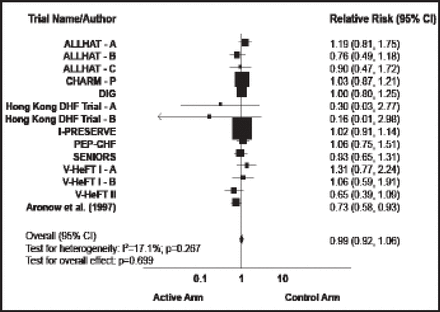
The second major failure is in HF with preserved EF (HF-PEF), which represents 33% to 50% of all HF patients. Two key trials in this population failed to show a benefit in death or HF hospitalization with treatment: the CHARM-Preserved trial of candesartan versus placebo (HR, 0.92; p=0.221) [MacDonald MR et al. Eur Heart J 2008] and the PEP-CHF trial of perindopril versus placebo (HR, 0.92; p=0.545) [Cleland JG et al. Eur Heart J 2006]. The I-PRESERVE trial found no benefit with the renin-angiotensin-aldosterone system blocker irbesartan versus placebo in death or hospitalization (HR, 0.95; p=0.35) [McMurray JJ et al. J Heart Fail 2007]. A meta-analysis of trials in patients with HF-PEF found that none of the treatments studied has a proven benefit in these patients (Figure 1) [Holland DJ et al. J Am Coll Cardiol 2011]. The ongoing TOPCAT trial [NCT00094302] is investigating the potential benefit of a treatment with an aldosterone antagonist in this condition.
Treatment Effect on Mortality.
Reproduced with permission from the American College of Cardiology. Effects of Treatment on Exercise Tolerance, Cardiac Function, and Mortality in Heart Failure With Preserved Ejection Fraction. Holland DJ et al. J Am Coll of Cardiol 2011;57(16):1676–1686.
New Drugs
New drugs being investigated for HF include renin inhibitors, such as aliskiren, inotropes, vasodilators/GMPc modulators, chimeric natriuretic peptides (NPs), neutral endopeptidase (NEP) inhibitors, and sinus-node inhibitors. The ATMOSPHERE trial [NCT00853658] is comparing treatment with aliskiren versus enalapril in 2162 patients. The PARADIGM-HF study [NCT01035255] is investigating LCZ696, a molecular complex of the ARB valsartan, and the NEP inhibitor AHU 377, in 7980 patients. The new vasodilator relaxin acts through nitric oxide and cyclic GMP effectors and has anti-inflammatory, anti-ischemic, anti-apoptotic, and antifibrotic properties. Results of proof-of-concept studies will determine if this drug has a place in the management of acute HF. Studies are also ongoing with the chimeric natriuretic peptide CD-NP.
Preliminary results of the HORIZON-HF [NCT00616161] trial show that the new calcium-cycling modulator istaroxime produced a rapid and sustained dose-dependent significant decrease in pulmonary capillary wedge pressure that was maintained at 6 hours. A proof-of-concept study of the direct myosin activator, omecamtiv mecarbil, showed improvement in heart function indices.
New Devices
Two trends in the use of devices for hemodynamic support are emerging: 1) the move toward using them as destination therapy rather than a bridge to transplantation, and 2) the development of new indications for CRT based on results from 2 studies. The REVERSE trial analyzed the percentage of mild to moderate HF patients with improved/unchanged or worsened condition with the CRT turned on or off [Daubert C et al. J Am Coll Cardiol 2009]. At 24 months, 81% of patients in the CRT ON group were improved/unchanged versus 66% in the CRT OFF group (p=0.01). Left ventricular end-systolic volume index was also significantly improved in the CRT ON group (69.7 mL/m2) versus the CRT OFF group (94.5 mL/m2; p<0.0001). The RAFT trial compared ICD versus ICD-CRT in patients with mild to moderate HF (n=1798) [Tang AS et al. N Engl J Med 2010]. Patients with ICD-CRT had significantly better all-cause mortality or HF hospitalization rates versus those with an ICD alone (HR, 0.56; p<0.001).
Other Approaches
Other approaches in HF management include cell therapy using cardiac skeletal muscle myoblast injections, embryonic or bone marrow stem cells, and gene therapy. The AGENT-HF trial is investigating gene therapy with percutaneous intracoronary infusion of SERCA2a, which has decreased expression in HF. Several preclinical studies indicate that restoring SERCA2a levels improves cardiac function. The CUPID trial [NCT00454818] in the United States is evaluating SERCA2a in HF.
The HF-ACTION trial investigated the effects of exercise training in patients with HF. Patients who received exercise training versus usual care had significantly decreased rates of CV death or HF hospitalization (adjusted HR, 0.85; 95% CI, 0.74 to 0.99; p=0.03) [O'Connor CM et al. JAMA 2009].
Telemonitoring allows physicians to remotely monitor parameters usually evaluated in an office visit, including a patient's weight and blood pressure. This technology alerts physicians to any significant variations in patients' parameters, and this approach might benefit patients with CHF.
- © 2012 MD Conference Express®