Summary
The focus this article is on a class of leukocyte adhesion molecules, leukocyte β2-integrins, among which Mac-1 (aMb2, CD11b/CD18) is the most common integrin on neutrophils. The leukocyte Mac-1 receptor interacts with the glyocoprotein Iba (GPIba) receptor on platelets, thereby regulating pro-inflammatory and pro-thrombotic bidirectional signals in both inflammatory cells and platelets.
- Inflammatory Disease
The focus of Dr. Daniel I. Simon's research at University Hospitals Harrington Heart & Vascular Institute, Case Western Reserve University School of Medicine, Cleveland, Ohio, USA, is on a class of leukocyte adhesion molecules, leukocyte β2-integrins, among which Mac-1 (αMb2, CD11b/CD18) is the most common integrin on neutrophils. The leukocyte Mac-1 receptor interacts with the glyocoprotein Ibα (GPIbα) receptor on platelets, thereby regulating pro-inflammatory and pro-thrombotic bidirectional signals in both inflammatory cells and platelets. Dr. Simon has centered his research on the structure, function, and signaling of Mac-1, identifying the Mac-1 binding site for GPIbα and developing tools to disrupt leukocyte-platelet complexes that promote vascular inflammation.
The repair response following vascular injury is an inflammatory process in which neutrophils and monocytes are rapidly recruited to sites of arterial injury, including stented human blood vessels. Neutrophils appear within hours; monocytes/macrophages predominate at 7 days. Inflammatory cells are the most abundant cells in the stented human intima for months following injury. The inflammatory cells enter the blood vessel through a 2-step process, involving selectin-mediated rolling and then firm adhesion and diapedesis through the integrin Mac-1.
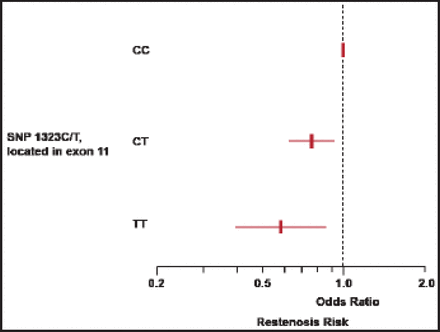
Inoue et al. [J Am Coll Cardiol 1996] observed that upregulation of the Mac-1 receptor on neutrophils predicts restenosis risk. Dr. Simon used Mac-1 knockout mice to prove the importance of the receptor in the vascular injury response [Simon DI et al. J Clin Invest 2000]. Following arterial injury, the wild-type mouse (Mac-1+/+) develops thick neointima while the Mac-1-/- mouse is protected from neointima growth. In stented rabbit arteries, brisk recruitment of inflammatory cells was disrupted by antibody targeting of the Mac-1 receptor, resulting in dramatically reduced restenosis [Rogers C et al. Proc Natl Acad Sci U S A 1998]. In humans, a single nucleotide polymorphism (SNP) in the CD18 locus of the β2 integrin is highly predictive of restenosis following stenting [Koch W et al. Am J Cardiol 2001] (Figure 1).
CD 18 Genetic Polymorphism Linked to Restenosis Risk.
Reproduced with permission from Elsevier. Koch W et al. Association of a CD18 gene polymorphism with a reduced risk of restenosis after coronary stenting. Am J Cardiol 2001;88(10):1120.
Mac-1 signaling via ligand engagement and clustering is important in amplifying the inflammatory response. Clustering of Mac-1 activates the master inflammatory transcription factor NFkB via a Toll/IL-1 receptor family-like signaling pathway [Shi et al. Circulation Research 2001]. Mac-1 signaling also regulates the expression of the transcription factor Foxp1 [Shi C et al. J Clin Invest 2004], which serves as a repressor of the gene encoding the M-CSF receptor. Mac-1 signaling downregulates the expression of Foxp1, thereby promoting monocyte differentiation and pro-inflammatory macrophage functions. Overexpression of Foxp1 specifically in monocytes/macrophages prevents monocyte maturation, resulting in reduced vascular inflammation and atherosclerosis.
The interaction between Mac-1 and platelet GPIbα broadly regulates inflammation in diverse animal models, including restenosis, vasculitis, glomerulonephritis, and demyelinating diseases. Dr. Simon is using these model systems to develop anti-inflammatory drugs for this diverse disease subset. Using chimeric integrins, his laboratory identified the 16-amino acid sequence within the I-domain of Mac-1 that is necessary and sufficient for Mac-1 binding to GPIbα. A peptide (M2) corresponding to this sequence or an antibody targeting this sequence (anti-M2) block Mac-1 binding to GPIbα but not to other Mac-1 ligands, including fibrinogen, ICAM-1, and JAM-3. Two of these amino acids, threonine 213 and arginine 216, are critical for binding GPIbα [Ehlers R et al. J Exp Med 2003].
The first approach to disrupting platelet binding to leukocytes in vivo was leveraging the anti-M2 antibody. Anti-M2 reduced neointimal thickening 28 days after injury and attenuated tissue injury responses in models of glomerulonephritis [Hirahashi J et al. Circulation 2009]. and demyelinating disease [Langer HF et al. Circulation Research 2012]. These and other studies strongly suggest that virtually all inflammation is platelet-dependent.
Understanding the molecular mechanisms of inflammatory cell recruitment and monocyte differentiation provides insights necessary to develop anti-inflammatory strategies for broadly modulating vascular injury.
- © 2012 MD Conference Express®