Summary
This article discusses the prognostic role of inflammatory biomarkers in heart failure (HF), their effect on left ventricular function and remodeling, and treatment strategies targeting inflammation in HF.
- Heart Failure Genomics
- Inflammatory Disease
Biykem Bozkurt, MD, PhD, Baylor College of Medicine, Houston, Texas, USA, discussed the prognostic role of inflammatory biomarkers in heart failure (HF), their effect on left ventricular (LV) function and remodeling, and treatment strategies targeting inflammation in HF. Numerous studies have documented elevated cytokines in patients with symptomatic HF. Studies have found an association between increased C-reactive protein (CRP), tumor necrosis factor-alpha (TNFα), and interleukin-6 levels, and poor survival in HF.
Cytokines in Heart Failure
Inflammatory mediators, which are activated following myocardial injury, correlate with disease severity and prognosis, and continue to exert deleterious effects on the heart. Possible sources of cytokines in HF include immune activation, myocardial biosynthesis, hypoperfusion of metabolic tissue, and altered distribution, degradation, and clearance. TNFα can be synthesized in the myocardium; adult cardiac myocytes express TNF following hemodynamic overload or stretch but not in normal hemodynamic loading conditions. TNFα is associated with reduced LV fractional shortening [Bozkurt B et al. Circulation 1998].
Soluble toll-like receptor-2 (ST2) is an interleukin-1 released by stretched myocytes. Serum ST2 levels significantly increase after acute myocardial infarction and inversely correlate with ejection fraction (EF). In patients with chronic HF, serum ST2 levels are associated with adverse outcomes [Rehman SU et al. J Am Coll Cardiol 2008]. Plasma levels of myeloperoxidase, an indirect marker of oxidative stress denoting leukocyte activation, correlate with HF severity and are an independent predictor of HF death [Tang WH et al. Am J Cardiol 2006].
Therapeutic Approaches
Targeting inflammation in HF patients has met with little success. An early small study demonstrated a significant increase in EF with prednisone treatment in patients with dilated cardiomyopathy (DCM) [Parrillo JE et al. N Engl J Med 1989]. Patients with active inflammation had a better response than nonreactive patients. Sliwa and colleagues [Circulation 2004] reported that DCM patients treated with pentoxifylline had significant improvements in EF and marked reductions in TNFα, CRP, and N-terminal prohormone of brain natriuretic peptide.
Following these studies, there was an intensive effort to develop targeted anticytokine therapy, especially targeting TNF. In the RENEWAL study, 900 patients with HF were randomly assigned to treatment with etanercept or placebo. The investigators found no difference in event-free survival after 96 weeks of treatment and speculated that targeting one cytokine might be too selective [Mann DL et al. Circulation 2004].
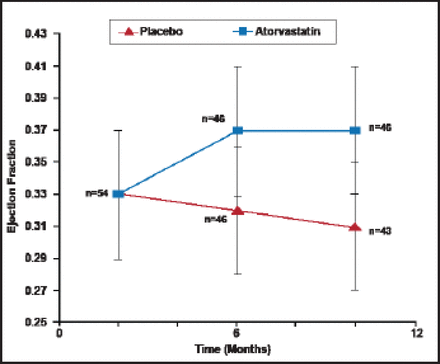
Treatment with beta-blockers significantly improves the cytokine profile in HF patients. Ohtsuka and colleagues [J Am Coll Cardiol 2001] demonstrated marked decreases in TNFα after beta-blocker treatment. Sola and colleagues [J Am Coll Cardiol 2006] reported marked reductions in TNFR2 levels after statin treatment in DCM patients (Figure 1).
Effect of Statin on Inflammatory Cytokines in Dilated Cardiomyopathy Patients.
Reproduced with permission from the American College of Cardiology. Salo S et al. Atorvastatin Improves Left Ventricular Systolic Function and Serum Markers of Inflammation in Nonischemic Heart Failure. J Am Coll Cardiol, Jan 17 2006;47(2):332.
Most inflammatory biomarkers are implicated in the pathogenesis of HF. Circulating levels correlate with HF severity and prognosis. The question remains as to whether inflammation should be targeted. Large-scale clinical trials have failed to demonstrate that treating inflammation improves clinical outcomes.
- © 2012 MD Conference Express®