Summary
This article reviews the key studies from the past year in 5 areas of cardiology: interventional, imaging, atrial fibrillation, antithrombotic agents for coronary artery disease and acute coronary syndrome, and lipid therapy.
- Coronary Artery Disease
- Lipid Disorders
- Interventional Techniques & Devices
- Imaging Modalities
- Arrhythmias
Gregory R. Giugliano, MD, SM, Tufts University School of Medicine and Baystate Medical Center, Springfield, Massachusetts, USA, reviewed the key studies from the past year in 5 areas of cardiology: interventional, imaging, atrial fibrillation (AF), antithrombotic agents for coronary artery disease (CAD) and acute coronary syndrome (ACS), and lipid therapy.
Interventional Cardiology
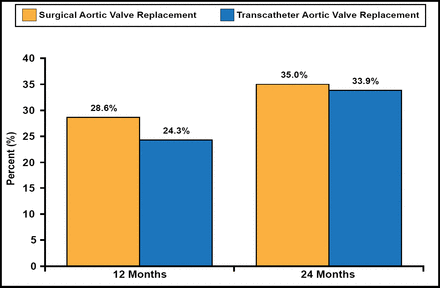
The multicenter, randomized Placement of Aortic Transcatheter Valve Trial [PARTNER; Smith CR et al. N Engl J Med 2011] Cohort A compared surgical aortic valve replacement (SAVR; n=351) with transcatheter aortic valve replacement (TAVR; n=348) in high-risk patients with severe stenosis. The primary endpoint was 1-year mortality from any cause.
The primary endpoint of 1-year all-cause mortality for SAVR was 26.8% versus 24.2% for TAVR (HR, 0.93; 95% CI, 0.7 to 1.22; noninferiority p=0.001; superiority p=0.62; Figure 1). The 2-year mortality rate with SAVR versus TAVR was not significantly different (35.0% vs 33.9%; Figure 1). At 30 days, neurological event rates (stroke and transient ischemic attack) were significantly higher in the TAVR group than with SAVR (5.5% vs 2.4%; p=0.04). One- and 2-year neurological event rates remained significantly higher with TAVR (8.3%, 11.2%) versus SAVR (4.3%, 6.5%; 1-year, p=0.04; 2-year, p=0.05). No significant difference in the number of overall strokes was observed between TAVR (n=24) and SAVR (n=20) at 36 months (HR, 1.22; 95% CI, 0.67 to 2.23; p=0.52). Moderate or severe paravalvular aortic regurgitation (AR) was more common with TAVR than with SAVR at 1 year (7.0% vs 1.9%; p<0.001) and 2 years (6.9% vs 0.9%; p<0.001). The presence of any AR after TAVR was associated with a doubling of late mortality (HR, 2.11; 95% CI, 1.43 to 3.10; p<0.001). After 1 year, overall costs and quality of life were similar in patients who had SAVR or transfemoral TAVR.
All-Cause Mortality.
The PARTNER B trial [Leon MB et al. N Engl J Med 2011] evaluated TAVR versus medical therapy in 358 inoperable patients with severe aortic stenosis. At 2 years, survival was significantly better with TAVR compared with medical therapy (67.6% vs 43.3%; p<0.0001). More strokes occurred in patients treated with TAVR.
Imaging: Coronary CT Angiography
The Rule Out Myocardial Ischemia/Infarction by Computer-Assisted Tomography [ROMICAT; Hoffmann U et al. N Engl J Med 2012] II trial compared coronary computed tomography angiography (CCTA) with standard evaluation in 1000 patients with acute chest pain. The patients were followed for 28 days. The primary endpoint was length of hospital stay. Secondary endpoints were time to diagnosis, healthcare utilization, major adverse cardiac events (MACE), cost-effectiveness, and rate of emergency department (ED) discharge.
The length of hospital stay was significantly shorter for patients randomized to CCTA compared with standard evaluation (23.2 vs 30.8 hours, respectively; p=0.0002). MACEs were similar between the groups (CCTA [n=2] vs standard evaluation [n=5]; p=0.37). Time to diagnosis was significantly reduced with CCTA compared with standard evaluation (10.4 vs 18.7 hours; p=0.0001). Patients receiving CCTA versus standard evaluation had more diagnostic tests (p<0.0001), more invasive angiography (p=0.04), and greater radiation exposure. ED costs were reduced with CCTA compared with standard evaluation (US $2053 vs US $2532; p<0.0001).
Atrial Fibrillation
ARISTOTLE
The randomized, double-blind, double-dummy Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation [ARISTOTLE] trial evaluated apixaban for the treatment of patients with AF and at least 1 additional risk factor for stroke [Lopes RD et al. Am Heart J 2010]. A total of 18,201 patients were randomly assigned to treatment with apixaban (5 mg, oral, BID; n=9120) or warfarin (target INR 2–3; n=9081). The primary outcome was stroke or systemic embolism (SE). The primary safety outcome was ISTH major bleeding.
The primary outcome of stroke or SE was significantly reduced in patients treated with apixaban compared with warfarin (1.27% vs 1.60% per year; HR, 0.79) [Granger CB et al. N Engl J Med 2011]. The rates of hemorrhagic stroke were significantly lower with apixaban than with warfarin (0.24% vs 0.47% per year; HR, 0.51; p<0.001). Ischemic or uncertain stroke and SE rates were not significantly different between arms. All-cause death rates were lower for patients treated with apixaban compared with those treated with warfarin (3.52% vs 3.94%; HR, 0.89; p=0.047).
ISTH major bleeding occurred significantly less often in patients randomized to apixaban compared with warfarin (2.13% vs 3.09% per year; HR, 0.69; p<0.001), a difference that was largely driven by a reduction in intracranial hemorrhage (ICH; 0.33% vs 0.80% per year; HR, 0.42; p<0.001). Major bleeding or clinically relevant non-major bleeding was significantly less in patients treated with apixaban compared with warfarin (4.07% vs 6.01% per year; HR, 0.68; p<0.001; Table 1).
Bleeding Outcomes.
PALLAS Trial
The aim of the Permanent Atrial Fibrillation Outcome Study Using Dronedarone on Top of Standard Therapy [PALLAS; Connolly SJ et al. N Eng J Med 2011] trial was to demonstrate the efficacy of dronedarone for preventing major cardiovascular (CV) events or unplanned CV hospitalization or all-cause death in patients with permanent AF and additional risk factors. Investigators randomly assigned 3236 patients to treatment with dronedarone (400 mg BID) or placebo with a follow-up of 3.5 months.
The study was terminated early because of safety concerns with dronedarone. The first coprimary outcome (stroke, SE, myocardial infarction [MI], or CV death) occurred in more patients treated with dronedarone compared with placebo (43 vs 19, respectively; HR, 2.29; p=0.002). The second coprimary outcome of unplanned hospitalization for CV causes or death also occurred in significantly more dronedarone-treated patients than placebo-treated patients (127 vs 67, respectively; HR, 1.95; p<0.001). Adverse events (AEs), serious AEs, and discontinuations because of AEs occurred more frequently with dronedarone versus placebo (p<0.001). Dronedarone restored sinus rhythm at 4 months in significantly more patients than placebo (p=0.001). At 1 month, dronedarone patients had significantly decreased heart rates (p<0.001) and systolic blood pressures (p=0.003) versus placebo patients.
Antithrombotic Agents in CAD/ACS
ATLAS ACS 2-TIMI 51 Trial
The Rivaroxaban in Combination With Aspirin Alone or with Aspirin and a Thienopyridine in Patients with Acute Coronary Syndromes [ATLAS ACS 2-TIMI 51] trial evaluated the efficacy and safety of rivaroxaban added to standard care in patients with recent ACS [Mega JL et al. N Engl J Med 2012]. A total of 15,526 patients were randomly assigned to treatment with rivaroxaban 2.5 mg BID (n=5174) or 5.0 mg BID (n=5176) or placebo (n=5176) plus standard care for a mean of 13 months.
The primary endpoint of CV death, MI, or stroke occurred in 8.9% of rivaroxaban (both doses combined) patients versus 10.7% of placebo patients (HR, 0.84; p=0.008). The very low dose 2.5-mg rivaroxaban group demonstrated significantly lower rates of CV death (2.7% vs 4.1%; HR, 0.66; p=0.002) and all-cause death (2.9% vs 4.5%; HR, 0.68; ITT p=0.002) compared with placebo-treated patients. These benefits were not observed in the 5-mg rivaroxaban group.
The combined rivaroxaban group compared with placebo treated patients had significantly higher rates of bleeding (noncoronary artery bypass graft TIMI major bleeding [2.1% vs 0.6%; HR, 3.96; p<0.001]; TIMI minor bleeding [1.3% vs 0.5%; p=0.003]; TIMI bleeding requiring medical attention [14.5% vs 7.5%; p<0.001]; ICH [0.6% vs 0.2%; p=0.009]). The very low dose 2.5-mg rivaroxaban group had significantly less bleeding compared with the 5.0-mg rivaroxaban group TIMI minor bleeding (0.9% vs 1.6%; p=0.046), TIMI bleeding requiring medical attention (12.9% vs 16.2%; p<0.001), and fatal bleeding (0.1% vs 0.4%; p=0.04).
Vorapaxar
Vorapaxar, the first oral thrombin receptor (PAR-1) antagonist was evaluated versus placebo in patients with ACS in the Trial to Assess the Effects of SCH 530348 in Preventing Heart Attack and Stroke in Patients with Acute Coronary Syndrome [TRACER; Tricoci P et al. N Engl J Med 2012]. No significant difference was observed for the primary endpoint of CV death, MI, stroke, hospitalization for ischemia, or urgent revascularization in patients treated with vorapaxar or placebo (18.5% vs 19.9%, respectively; HR, 0.92; p=0.072). Global Use of Strategies to Open Occluded Coronary Arteries (GUSTO) moderate or severe bleeding was significantly higher for patients treated with vorapaxar compared with placebo (7.2% vs 5.2%, respectively; HR, 1.35; p<0.001).
In the Trial to Assess the Effects of SCH 530348 in Preventing Heart Attack and Stroke in Patients with Atherosclerosis [TRA 2°P-TIMI 50; Morrow DA et al. N Engl J Med 2012], the primary endpoint (composite of CV death, MI, or stroke) occurred less frequently in patients treated with vorapaxar compared with placebo (9.3% vs 10.5%, respectively; HR, 0.87; p<0.001). When compared with placebo treatment, patients treated with vorapaxar had significantly more moderate or severe bleeding (2.5% vs 4.2%; HR, 1.66; p<0.001) and ICH (0.5% vs 1.0%; p<0.001).
Duration of Dual Antiplatelet Therapy Post Stenting
The Synergy Between Stent and Drugs to Avoid Ischemic Recurrences After Percutaneous Coronary Intervention [PRODIGY; Valgimigli M et al. Circulation 2012] trial assessed 6 versus 24 months of dual antiplatelet therapy (DAPT) with aspirin and clopidogrel after coronary stenting. At 2 years, the primary composite endpoint (death, MI, or stroke) rates were 10.1% with 6-month treatment versus 10.0% with 24-month treatment (p=0.91). Patients receiving long-term DAPT had twice the risk of bleeding (HR, 2.17; 95% CI, 1.44 to 3.22; p=0.00018) and transfusion (HR, 0.50; 95% CI, 0.26 to 0.98; p=0.041).
Lipid Therapy
The Niacin Plus Statin to Prevent Vascular Events [AIM HIGH; AIM HIGH Investigators. N Engl J Med 2011] trial evaluated stable patients with vascular disease who were all on a background of simvastatin therapy and were randomly assigned to the addition of niacin versus placebo. There was no difference in the primary endpoint (combination of coronary heart disease death, MI, ischemic cerebrovascular accident, ACS, or revascularization) between the niacin group compared with placebo (16.4% vs 16.2%; p=0.79). There was a trend toward more ischemic stroke in patients treated with niacin compared with placebo (p=0.11). The study was stopped early by the independent data safety monitoring committee because of futility.
Proprotein convertase subtilisin/kexin type 9 (PCSK9) is a proteolytic enzyme that plays a key role in the degradation of the LDL receptor (LDL-R). Decreased PCSK9 levels lead to increased availability of LDL-Rs, thereby reducing LDL-C levels. In a Phase 2 dose-finding study of a new agent that is an antibody to PCSK9, SAR236553/REGN727 showed that this new class of lipid-lowering therapy could lower LDL-C levels by up to 72% after 12 weeks of therapy [McKenney JM et al. J Am Coll Cardiol 2012].
- © 2012 MD Conference Express®