Summary
Every year, 17.1 million lives are claimed by the global burden of heart disease and stroke, 82% of which are in the developing world [World Heart Federation. State of the Heart. Cardiovascular Disease Report 2012]. In the United States, cardiovascular disease is the leading cause of death, responsible for 17% of national health expenditures.
- Lipid Disorders
Lipid Management. Is It All About Numbers?
Every year, 17.1 million lives are claimed by the global burden of heart disease and stroke, 82% of which are in the developing world [World Heart Federation. State of the Heart. Cardiovascular Disease Report 2012]. In the United States, cardiovascular disease (CVD) is the leading cause of death, responsible for 17% of national health expenditures. By 2030, an aging population and other risk factors [Kones R. Drug Des Devel Ther 2011] are expected to drive that figure to $276 billion [Heidenreich PA et al. Circulation 2011].
Raul D. Santos, MD, PhD, University of São Paulo, São Paulo, Brazil, discussed the role of lipid management in CVD. Topics covered during his presentation included the importance of cholesterol in CVD; intervention studies with statins; the meaning of lower low-density lipoprotein cholesterol (LDL-C) and non–high-density lipoprotein cholesterol (HDL-C) levels; the ideal cholesterol level; the cost-effectiveness of LDL-C lowering; and the relation between the use of statins and the potential risk for type 2 diabetes, hemorrhagic stroke, and severe muscle damage.
The Importance of Cholesterol for CVD
The Prospective Studies Collaboration [Lancet 2007] demonstrated that a 1 mmol/L decrease in total cholesterol was associated with about one-half (HR, 0.44; 95% CI, 0.42 to 0.48), one-third (HR, 0.66; 95% CI, 0.65 to 0.68), and one-sixth (HR, 0.83; 95% CI, 0.81 to 0.85) lower ischemic heart disease in both sexes at ages 40 to 49, 50 to 69, and 70 to 89 years, respectively, and that the absolute effects of cholesterol and blood pressure were approximately additive.
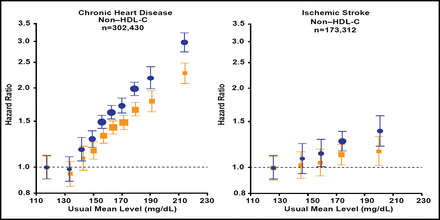
Among the major lipids and apolipoproteins in vascular risk, the hazard ratio for coronary heart disease (CHD) with non–HDL-C is approximately 4 times greater than that for ischemic stroke [Di Angelantonio E et al. JAMA 2009] (Figure 1); in the Anglo-Scandinavian Cardiac Outcomes Trial—Lipid Lowering Arm [ASCOT-LLA], there were 100 primary events in the atorvastatin group versus 154 in the placebo group (HR, 0.64; 95% CI, 0.50 to 0.83; p=0.005) [Sever PS et al. Lancet 2003].
Non–HDL-Cholesterol, CHD, and Ischemic Stroke.
Follow-up 2.79 million per person years; CHD=coronary heart disease; HDL=high-density lipoprotein.
Reprinted from JAMA 2004;292(20):2471. Di Angelantonio E. et al. Obesity an th risk of new onset atrial fibrillation; with permission from the American Medical Association.
Statins produce significant reductions in cholesterol. The Statin Therapies for Elevated Lipid Levels Compared across Doses to Rosuvastatin [STELLAR] trial showed that across dose ranges, rosuvastatin decreased total cholesterol significantly more (p<0.001) than all comparators [Jones PH et al. Am J Cardiol 2003].
Data from the Cholesterol Treatment Trialists' (CTT) Collaboration [Lancet 2010] suggest that reduction of LDL-C by 2 to 3 mmol/L can reduce risk of heart attack, revascularization, and ischemic stroke by about 40% to 50%. Law et al. [BMJ 2003] demonstrated that statins can lower LDL-C concentration by an average of 1.8 mmol/L, reducing the risk of ischemic heart disease events by 60% and stroke by 17%.
Non–HDL-C is the second lipid target of therapy after LDL-C. The relationship between percent non–HDL-C lowering and CHD reduction is approximately 1:1 [Robinson JG et al. J Am Coll Cardiol 2009].
Potential Risks of Statins
Myalgias (including infrequent cases of rhabdomyolysis) and elevation in transaminases have been reported for all statins. New data with simvastatin indicate that co-administration of medications that interfere with the metabolism of simvastatin by the cytochrome P450 enzyme increase the risk of rhabdomyolysis [Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine (SEARCH) Collaborative Group. Lancet 2011]. These findings led to an advisory from the FDA to adjust downward the dose of simvastatin when used concomittantly with certain calcium channel blockers, ranolazine, and amiodarone, and to avoid the use of simvastatin 80 mg in most patients [http://www.fda.gov/Drugs/DrugSafety/ucm256581.htm].
Although epidemiologic evidence indicates an inverse association between LDL-C and incident cancer, statins are not associated with an increased risk of cancer [Alsheikh-Ali AA et al. J Am Coll Cardiol 2008]. These findings have been corroborated in the CTT Collaboration [Lancet 2010]. Thus the epidemiologic data appear to be confounded by other factors associated with cancer (eg, malnutrition) that cause low LDL-C.
Statin therapy is associated with a slightly increased risk of incident diabetes. Sattar et al. [Lancet 2010] found 1 extra case after treating 255 (95% CI, 150 to 852) patients with statins for 4 years. Preiss et al. [JAMA 2011] identified 2 additional cases in an intensive-dose group per 1000 patient-years. Waters et al. [J Am Coll Cardiol 2011] found a slightly increased risk of new-onset type 2 diabetes with high-dose atorvastatin treatment compared with placebo in the Stroke Prevention by Aggressive Reduction in Cholesterol Levels [SPARCL] trial. Risk factors across 3 large randomized clinical trials included baseline fasting glucose level and features of metabolic syndrome.
Based on these findings, Dr. Santos concluded that there is a clear association between cholesterol levels and the risk of CHD death, that cholesterol lowering reduces the risk of cardiovascular events and mortality without increasing the risk of cancer, and that statin use is associated with an increase in the risk of type 2 diabetes. However, the absolute risk is small and is greater in those at higher risk of becoming diabetic at baseline.
2012 Update on Intensive Lipid Lowering
Robert P. Giugliano, MD, SM, Harvard Medical School, Boston, Massachusetts, USA, provided an update on intensive lipid lowering. His presentation included a rationale for it and a review on the efficacy of statins. He also discussed their use in selected populations, new safety concerns, and future therapies to reduce LDL-C.
A key question is how low should we go? Data suggest that the incidence of cardiovascular events would approach zero if LDL-C were <60 mg/dL in primary prevention and approximately 30 mg/dL in secondary prevention [Hochholzer W & Giugliano RP. Ther Adv Cardiovasc Dis 2010].
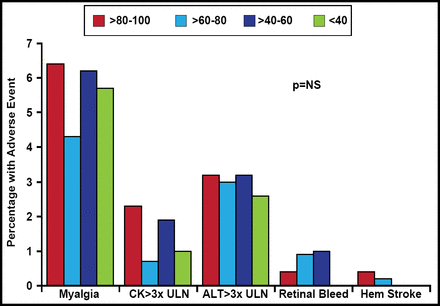
Evidence indicates that such goals are not beyond the realm of possibility. In the CTT Collaboration [Lancet 2010], additional reductions in LDL-C safely produced further decreases in the incidence of heart attack, revascularization, and ischemic stroke with no evidence of any threshold within the cholesterol range studied. Wiviott et al. [J Am Coll Cardiol 2005] reported no intrinsic safety concerns with a strategy of intensive statin treatment to <40 mg/dL LDL (Figure 2).
The Safety of Achieving Low LDL-C Goals.
ALT=alanine aminotransferase; CK=creatine kinase; LDL-C=low-density lipoprotein cholesterol; NS=nonsignificant; ULN=upper limit of normal.
Reprinted from J Am Coll Cardiol 2005;46(8):1411. Wiviott SD et al. Can low density lipoprotein be too low? With permission from Elsevier.
Statins in Selected Populations
In an apparently healthy population of individuals without hyperlipidemia but with elevated high-sensitivity C-reactive protein, statin treatment reduced hazard ratios for all-cause death by 20% (p=0.02), 54% for MI (p=0.0002), 48% for stroke (p=0.002), 46% for arterial revascularization (p<0.0001), and 47% for CV death, MI, or stroke (p<0.00001) [Ridker PM et al. N Engl J Med 2008].
Rosuvastatin significantly decreased CVD events in a similar population of women, with relative reductions akin to those in men. A corroborating meta-analysis of women in primary prevention trials showed a one-third reduction in risk of CV death [Mora S et al. Circulation 2010].
In patients undergoing hemodialysis, treatment with rosuvastatin lowered LDL-C levels but had no significant effect on the composite primary endpoint of death from cardiovascular causes, nonfatal MI, or nonfatal stroke [Fellstrom BC et al. N Engl J Med 2009]. Findings were the same in an earlier atorvastatin study [Wanner C et al. N Engl J Med 2005].
New Safety Concerns
An FDA safety alert (February 28, 2012) warned of reversible cognitive impairment with statin treatment. However, data from observational studies and clinical trials do not suggest that cognitive changes associated with statin use are common or lead to clinically significant cognitive decline [http://www.fda.gov].
Hsia et al. [J Am Coll Cardiol 2011] assessed the impact of LDL-C levels <50 mg/dL on CV and adverse events in a population of apparently healthy adults treated with rosuvastatin. Outcomes showed that rates of myalgia, muscle weakness, neuropsychiatric conditions, cancer, and diabetes were not significantly different among treated individuals with and without LDL-C <50 mg/dL; memory loss was not a significant side effect (p=0.041).
Extended follow-up in the Heart Protection Study (11.0 years; SD 0.6) demonstrated that more prolonged LDL-lowering statin treatment produced larger absolute reductions in vascular events. Even after the end of study treatment with simvastatin, benefits persisted for at least 5 years without any evidence of emerging hazards [Heart Protection Study Collaborative Group. Lancet 2011].
Future Therapies to Reduce LDL
Dual inhibition attacks cholesterol production (statin) and absorption (ezetimibe), leading to a reduction in hepatic cholesterol stores and increasing clearance of cholesterol from the blood. Winkler et al. [Atherosclerosis 2011] found that ezetimibe alone and in combination with simvastatin significantly reduced the concentration of atherogenic small, dense LDL-C in patients with type 2 diabetes.
Cholesterol ester transfer protein (CETP) inhibition is another promising approach. CETP is a plasma protein that catalyzes the transfer of cholesterol ester from HDL to apoB-containing lipoproteins (very LDL-C and LDL-C) in exchange for triglycerides. A major study on this treatment approach will conclude in 2017 [www.revealtrial.org].
Biologics also show potential therapeutic value. Proprotein convertase subtilisn/kexin type 9 (PCSK9) plays an important role in degrading LDL-receptor (LDL-R). The resulting decrease in PCSK9 leads to an increase in LDL-R and lower LDL-C levels [Garber K. Nat Biotech 2012]. McKenney et al. [J Am Coll Cardiol 2012] have demonstrated that, when added to atorvastatin, PCSK9 inhibition with SAR236553 further reduces LDL-C by 40% to 72%, with additional dose- and dosing frequency-dependent declines.
Based on these data, Dr. Giugliano concluded that lowering LDL with statins reduces mortality and CV events; that statins are effective in primary prevention and women, with more modest benefits in patients with chronic kidney disease; that statins do not raise the risk of cancer but modestly increase incident diabetes mellitus; that the role of statins in cognitive impairment remains to established; and that future therapies to lower LDL include ezetimibe, CETP inhibitors, and PCSK9 inhibitors.
The editors would like to thank the many members of the Caribbean Cardiac Society presenting faculty who generously gave their time to ensure the accuracy and quality of the articles in this publication.
- © 2012 MD Conference Express®