Summary
This article presents key results from the first Phase 3 trial of certolizumab pegol (CZP) in patients with active psoriatic arthritis (PsA). In the Phase 3, Multicenter, Randomized, Double-Blind, Parallel Group, Placebo-Controlled Study to Evaluate the Efficacy and Safety of Certolizumab Pegol in Subjects With Adult Onset Active and PsA study [RAPID-PsA; NCT01087788], patients who were treated with CZP were twice as likely to meet the primary study endpoint of ACR20 response at Week 12 compared with those who were taking placebo.
- Arthritis Clinical Trials
Philip J. Mease, MD, University of Washington, Seattle, Washington, USA, presented key results from the first Phase 3 trial of certolizumab pegol (CZP) in patients with active psoriatic arthritis (PsA). In the Phase 3, Multicenter, Randomized, Double-Blind, Parallel Group, Placebo-Controlled Study to Evaluate the Efficacy and Safety of Certolizumab Pegol in Subjects With Adult Onset Active and PsA study [RAPID-PsA; NCT01087788], patients who were treated with CZP were twice as likely to meet the primary study endpoint of ACR20 response at Week 12 compared with those who were taking placebo.
Dr. Mease presented the 24-week results from an ongoing 158-week randomized, placebo-controlled study that included 409 patients with active PsA who had failed 1 or more disease-modifying antirheumatic drugs, including a maximum of 1 anti-tumor necrosis factor (anti-TNF). Patients were randomized to placebo (n=136) or started on a loading dose of 400 mg CZP every 2 weeks for the first 4 weeks and then continued with either 200 mg CZP administered subcutaneously every 2 weeks (n=138) or 400 mg CZP administered subcutaneously every 4 weeks (n=135). Patients in the placebo group who failed to achieve a ≥10% improvement in tender joint count (TJC) and swollen joint count (SJC) at Weeks 14 and 16 were randomized to 1 of the CZP arms, following a loading dose. The primary efficacy endpoints were ACR20 response at Week 12 and change from baseline in the modified Total Sharp Score (mTSS) at Week 24 (mTSS results were not reported at the meeting). Key secondary endpoints included: ACR20 response and change from baseline in Health Assessment Questionnaire-Disease Index (HAQ-DI) at Week 24, the Psoriasis Area and Severity Index (PASI) 75 response at Week 24 in the subgroup of subjects with psoriasis involving ≥ 3% body surface affected (BSA) at baseline, and change from baseline in mTSS at Week 48.
Subjects were aged 47 to 48 years, evenly divided between men and women, and overweight (body mass index 29.2 to 30.5 kg/m2). Approximately 20% of patients had prior anti-TNF exposure. Subjects had significant disease, as evidenced by the SJC (mean 10.4 to 11.0/66), TJC (mean 19.6 to 21.5/68) the presence of enthesitis in >60% of subjects and dactylitis in approximately one-third of subjects. The median baseline PASI score for subjects was 7.0 to 8.1.
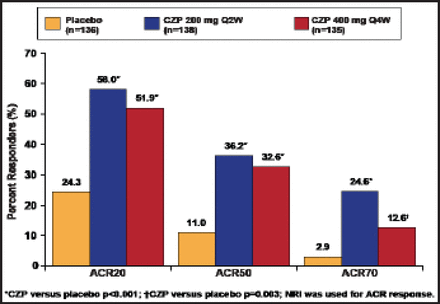
At Week 12, 58% of patients who received CZP 200 mg every 2 weeks and 51.9% of those who received CZP 400 mg every 4 weeks achieved an ACR20 response compared with 24.3% of patients who were receiving placebo (p<0.001). More CZP-treated patients in both groups also achieved ACR50 and ACR70 response compared with placebo (Figure 1). Results were observed as early as Week 1. Functional improvement was significant (p<0.001) for subjects who received CZP, with both groups reporting changes in HAQ-DI scores at Week 24 that exceeded the minimally important difference of 0.35 [Mease P et al. J Rheumatol 2011]. Although the response was slightly slower, treatment with CZP was associated with a robust skin response (Table 1).
ACR Response at Week 12.
PASI 75 and 90 Response.
The safety profile of CZP was similar to that observed in patients with rheumatoid arthritis. Adverse events occurred at rates of 68% for the placebo group versus 69% for the combined CZP group, and serious adverse events occurred at 4% for the placebo group versus 7% for the combined CZP group. Two deaths occurred during this phase of the study, but neither was considered to be related to the study drug.
- © 2012 MD Conference Express®