Summary
This article discusses the first results from the Autologous Stem Cell Transplantation International Scleroderma trial [ASTIS; ISRCTN54371254], an international, investigator-initiated, open-label Phase 3 trial that suggests that hematopoietic stem cell transplantation results in better long-term event-free and overall survival compared with conventional treatment for patients with poor prognosis, early diffuse, cutaneous systemic sclerosis.
- Rheumatology Clinical Trials
- Rheumatological Autoimmune Disorders
- Featured Meeting - Specialty page
Jacob M. van Laar, MD, Newcastle University, Newcastle, United Kingdom, presented the first results from the Autologous Stem Cell Transplantation International Scleroderma trial [ASTIS; ISRCTN54371254], an international, investigator-initiated, open-label Phase 3 trial that suggests that hematopoietic stem cell transplantation (HSCT) results in better long-term event-free and overall survival compared with conventional treatment for patients with poor prognosis, early diffuse, cutaneous systemic sclerosis.
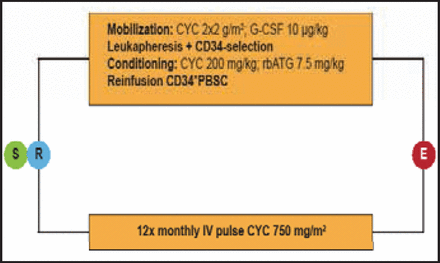
To be eligible for inclusion in the study, patients were required to be aged 16 to 65 years and have early, poor prognosis, diffuse, cutaneous systemic sclerosis, defined as a disease duration ≤4 years with a skin score ≥15 and evidence of heart, lung, or kidney involvement; or a disease duration ≤2 years with a skin score ≥20 and evidence of an acute-phase response. Patients with pulmonary hypertension >50 mm Hg or serious organ dysfunction were excluded, as were those with previous extensive (>5 gr IV; >3 months oral) treatment with cyclophosphamide (CYC). A total of 156 patients (mean age 43.7 years, 59% women, mean disease duration 1.4 years) were enrolled from 27 centers in 10 countries. Subjects were randomized to the HSCT arm (n=79) or to intravenous pulse CYC 750 mg/m2 (CYC-treated group; n=77; Figure 1) and monitored every 3 months up to 24 months and annually thereafter. The primary endpoint of the trial was event-free survival, defined as overall survival or survival until development of major organ failure.
Randomization.
CYC=cyclophosphamide; G-CSF=granulocyte colony-stimulating factor; rbATG=rabbit antithymoglobulin; PBSC=peripheral blood stem cell.
Sixty-one transplant patients and 54 CYC patients completed 24 months of follow-up. As of the time of this presentation, 15 patients in the treatment arm and 12 CYC patients had completed 84 months of follow-up, and 43 transplant subjects and 33 controls were still being followed. As of May 1, 2012, 46 events had occurred (19 in the transplant arm, and 27 in the CYC arm). Sixteen subjects in the transplant arm died compared with 26 deaths in the CYC arm. Half of the deaths in the HSCT group occurred early and were deemed to be treatment-related, according to an independent data-monitoring committee. In the conventional treatment group, none of the deaths was deemed to be treatment-related, but more deaths occurred later, and most were related to disease progression, cancer, and major organ failure.
Long-term (≥2 years) event-free and overall survival were significantly improved with HSCT treatment (HR, 0.30; 95% CI, 0.12 to 0.76; p=0.011 and HR, 0.22; 95% CI, 0.08 to 0.58; p=0.002, respectively).
Preliminary results of an exploratory analysis showed that smoking status appeared to be a determinant of event-free survival, with nonsmokers benefiting most from transplantation.
- © 2012 MD Conference Express®