Summary
Patients with osteoporosis who discontinue denosumab have transient increases in bone remodeling and decreases in bone mineral density [Miller PD et al. Bone 2008; Bone HG et al. J Clin Endocrinol Metab 2011], but the effect of discontinuation on fracture risk is not clear. The Phase 3 Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months [FREEDOM; NCT00089791] trial determined the incidence of fractures in postmenopausal patients with osteoporosis after stopping denosumab therapy.
- Metabolic Bone Disease
The Phase 3 Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months [FREEDOM; NCT00089791] trial demonstrated that postmenopausal women with osteoporosis who were treated for 3 years with denosumab (60 mg every 6 months) had a decreased risk of new vertebral, nonvertebral, and hip fractures compared with patients who received placebo for the same duration [Cummings SR et al. N Engl J Med 2009]. Patients with osteoporosis who discontinue denosumab have transient increases in bone remodeling and decreases in bone mineral density (BMD) [Miller PD et al. Bone 2008; Bone HG et al. J Clin Endocrinol Metab 2011], but the effect of discontinuation on fracture risk is not clear. The objective of this analysis, presented by Ove Törring, MD, PhD, Karolinska Institutet, Stockholm, Sweden, was to determine the incidence of fractures in postmenopausal patients with osteoporosis after stopping denosumab therapy.
The analysis included patients from the FREEDOM trial who received 2 to 5 doses of denosumab (n=327) or placebo (n=470) and discontinued treatment but remained in the study for at least 6 months after the last dose and the 1-month study visit window (≥7 months). The patients were observed while off treatment for a period of 6 to 24 months, beginning 7 months after the last dose.
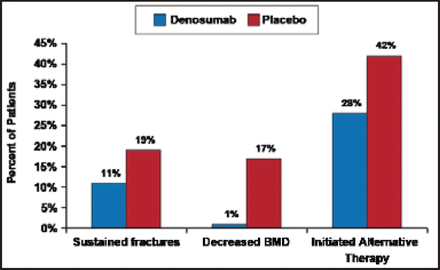
Baseline characteristics, including age, prevalent fracture, and lumbar spine and total hip BMD T-scores were similar between the 2 treatment groups. During the treatment period, 19% of placebo patients versus 11% of denosumab patients sustained fractures. More placebo patients had significantly decreased BMD (17%) versus denosumab patients (1%). After the last study drug dose, 42% of placebo patients and 28% of denosumab patients initiated alternative therapy (Figure 1).
Results
BMD=bone mineral density.
After stopping treatment, 9% of placebo patients and 7% of denosumab patients sustained a new fracture, with a fracture rate per 100 subject-years of 13.5 in the placebo group versus 9.7 in the denosumab group (HR, 0.82; 95% CI, 0.49 to 1.38), adjusted for age and total hip BMD T-score at baseline. Fracture rates during the off-treatment period were similar in the 2 groups.
The authors concluded that patients had no excess fracture risk after stopping denosumab compared with placebo patients during the off-treatment period, for up to 2 years.
- © 2012 MD Conference Express®