Summary
This article discusses studies of Janus kinase (JAK) inhibitors in the treatment of patients with rheumatoid arthritis. JAK inhibitors have short half-lives and can be administered orally once or twice daily. As of 2012, more than 50 clinical trials of JAK inhibitors have been completed or are ongoing. Also reviewed is the role of new orally bioavailable kinase inhibitors in the treatment of RA, and whether therapy will be influenced by their efficacy, safety profile, cost, and ability to be used with RA-related medications.
- Inflammatory Disorders
- Rheumatoid Arthritis
JAK Inhibition in RA
The Janus kinase (JAK) tyrosine kinase family consists of small intracellular molecules that are required for cytokine and growth factor signaling. Four members of the JAK family have been identified: JAK1, JAK2, JAK3, and Tyk2. JAK1 and JAK2 mediate the signals of cytokine targets in inflammatory diseases, such as rheumatoid arthritis (RA), while JAK3 is primarily involved in T cell-mediated immune function.
Joel M. Kremer, MD, Albany Medical College, Albany, New York, USA, discussed studies of JAK inhibitors in the treatment of patients with RA. JAK inhibitors have short half-lives and can be administered orally once or twice daily. As of 2012, more than 50 clinical trials of JAK inhibitors have been completed or are ongoing. Ruxolitinib is the first United States Food and Drug Administration-approved JAK inhibitor to be indicated for the treatment of myelofibrosis. A JAK1/2 inhibitor, ruxolitinib also has been tested in Phase 2 studies for RA. Other JAK inhibitors that are under investigation in RA include baricitinib, tofacitinib (CP-690,550), VX-509, and GLPG0634 (Table 1).
JAK Inhibitors Under Investigation.
Tofacitinib is a relatively selective JAK1/2/3 inhibitor that has been studied as monotherapy [Fleischmann et al. Arthritis Rheum 2012] and combined with disease-modifying antirheumatic drugs (DMARDs) [Kremer et al. Arthritis Rheum 2012]. In the Phase 2b dose-ranging study [Fleischmann et al. Arthritis Rheum 2012], patients were randomized to 1, 3, 5, 10, or 15 mg oral tofacitinib twice daily for 24 weeks, placebo for 24 weeks, or adalimumab 40 mg every other week for 12 weeks, followed by tofacitinib 5 mg twice daily for 12 weeks. The safety results showed that transaminase values were increased in the 5- and 10-mg tofacitinib groups, low-density lipoprotein cholesterol was increased in 29% of patients in the 5-mg group and 36% of patients in the 10-mg group; 6 patients experienced a confirmed 50% increase in serum creatinine, and 6 patients experienced confirmed severe anemia. A Phase 2 study of tofacitinib plus methotrexate demonstrated sustained efficacy at 5, 10, and 15 mg twice daily over 24 weeks.
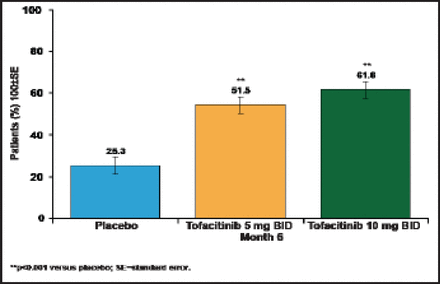
The Phase 2 data on tofacitinib supported investigation of the 5- and 10-mg doses in several Phase 3 monotherapy and combination therapy trials. The ORAL Scan trial [NCT00960440] evaluated tofacitinib 5 and 10 mg in combination with methotrexate (n=750). The 1-year interim analysis showed that at 6 months, the primary endpoint of ACR20 was achieved by 61.8% of tofacitinib 10 mg patients and 51.5% of tofacitinib 5 mg patients versus 25.3% of placebo patients (p<0.00001; Figure 1). Patients who were treated with tofacitinib 10 mg versus placebo had significantly greater reductions in HAQ-DI at 3 months (p<0.0001), and a greater percentage of patients achieved DAS28–4 (ESR) scores <2.6 at 6 months (p<0.0001). In a secondary analysis, both 5- and 10-mg tofacitinib doses were superior to placebo in the percentage of patients without radiographic progression.
1-Year Interim Analysis: ORAL Scan.
In the ORAL Step study [NCT00856544] of tofacitinib plus methotrexate (n=400), ACR20 response rates were significantly higher with tofacitinib 5 mg (p≤0.05) and 10 mg (p<0.0001) versus placebo at 3 months.
The Phase 3 trials of tofacitinib have demonstrated consistent efficacy and adverse events profiles. Tofacitinib is furthest along in the development process, but other JAK targets may offer a better efficacy/toxicity profile.
Small-Molecule Kinase Inhibitors in RA Treatment
The role of new orally bioavailable kinase inhibitors in the treatment of RA will be influenced by their efficacy, safety profile, cost, and ability to be used with RA-related medications. Iain B. McInnes, PhD, University of Glasgow, Glasgow, Scotland, United Kingdom, reviewed the available data on the use of these novel agents in the management of RA.
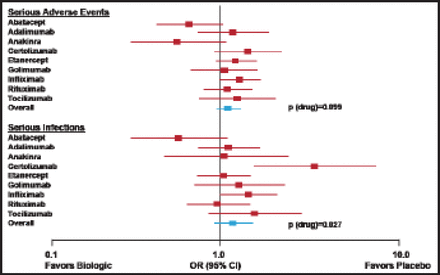
Several tumor necrosis factor (TNF) inhibitors have demonstrated efficacy in RA trials, including etanercept, infliximab, adalimumab, certolizumab, and golimumab. Abatacept, rituximab, and tocilizumab have been shown to improve ACR responses in RA patients who had an inadequate response to TNF inhibitors. Many of these agents are associated with of serious adverse events and infections [Singh J et al. Cochrane Database Syst Rev 2011] (Figure 2). Data from several registries demonstrate an increased relative risk of infection shortly after the start of TNF inhibitor treatment [Askling J et al. Curr Opin Rheum 2008]. Several randomized clinical trials found an increased risk of cancer in patients who were treated with anti-TNF therapies, with ORs ranging from 1.3 to 3.3.
Adverse Event/Serious Infection Profiles.
Reproduced with permission from I.B. McInnes, PhD.
Patients with RA often have comorbidities, including cardiovascular disease, insulin resistance, low bone mineral density, and depression. Greenberg et al. [Ann Rheum Dis 2011] reported that compared with nonbiologic disease-modifying antirheumatic drugs (DMARDs), the HRs for cardiovascular risk are 0.39 (95% CI, 0.19 to 0.82) with anti-TNF treatment and 0.94 (95% CI, 0.49 to 1.80) with methotrexate. With no treatment as a reference, treatment with prednisone yielded HR=1.78 (95% CI, 1.06 to 2.96) with <7.5 mg daily and HR=2.62 (95% CI, 1.29 to 5.31) with ≥7.5 mg daily.
With rigorous application of current medicines and advanced strategies, new therapies for RA have the potential to improve symptoms, signs, and function; reduce or eliminate damage; have an acceptable safety profile; have widespread patient endorsement; and increase adherence to target-based strategic approaches. These therapies could provide greater convenience, increased remission potential, cost-effectiveness, and/or durability.
Treatment strategies that are currently in development for RA include targeting DAMPs/PAMPs and other innate receptors, cytokines (eg, GM-CSF receptor, IL-17A, BlyS), protease-activated receptors (eg, PAR2), complement (eg, C5), kinase inhibitors (eg, SYK, JAK, BTK, PI3K), post-translational modification (eg, PADI4), and autoreactivity (eg, T-cell, β-cell). The most advanced kinase targets in RA are JAK and SYK. Both modulate inflammation and damage and are undergoing investigation in Phase 3 trials. Thus far, the efficacy datasets are encouraging. However, safety datasets will be a major driver in clinical decision-making. Additionally, questions remain about the effect of kinase inhibitors on comorbidities. Other factors that will affect the adoption of these new agents include patients' preferences (parenteral vs oral therapeutics) and cost-effectiveness.
Considerations for introducing kinase inhibitors in practice include comparing their tolerability and safety, efficacy, ease of administration, cost, predictability of responses, and pathological rationale with other treatments that are used in specific settings: premethotrexate (vs methotrexate, triple therapy), postmethotrexate inadequate response (IR; vs TNF inhibitor or other mechanism of action), and post-TNF inhibitor-IR (vs other biologic mechanism of action).
- © 2012 MD Conference Express®