Summary
Data from a Phase 2b study suggest that baricitinib, an oral JAK1/JAK2 inhibitor, is an efficacious treatment for rheumatoid arthritis when used in combination with traditional disease-modifying antirheumatic drugs.
- Rheumatoid Arthritis Clinical Trials
Data from a Phase 2b study, presented by Edward Keystone, MD, University of Toronto, Toronto, Ontario, Canada, suggest that baricitinib, an oral JAK1/JAK2 inhibitor, is an efficacious treatment for rheumatoid arthritis (RA) when used in combination with traditional disease-modifying antirheumatic drugs (DMARDs).
JAK tyrosine kinases differentially mediate signal transduction for a variety of cytokines that are involved in inflammatory conditions. JAK1 and JAK2 particularly affect IL-6, IL-12, IL-23, INF-γ, and INF-α/β, suggesting that inhibition of JAK1 or JAK2 would lead to immunomodulation or perhaps immunosuppression. This was a double-blind, placebo-controlled, randomized study to assess the effect of 4 doses of baricitinib, a potent, reversible inhibitor of JAK1 and JAK2, in patients with RA. Eligible subjects had ≥8 swollen and ≥8 tender joints, based on the 66/68 joint count; were on a stable dose of methotrexate for at least 12 weeks or corticosteroids for 6 weeks; and had a maximum C-reactive protein (CRP) level >1.2x the upper limit of normal or an erythrocyte sedimentation rate >28 mm/hr. Subjects were randomized to placebo (n=98) or baricitinib 1, 2, 4, or 8 mg daily (∼50 subjects each) in combination with methotrexate and treated for 12 weeks. The primary study endpoint was the ACR20 response at Week 12 for the combined 4- and 8-mg dose groups versus placebo. Major secondary endpoints included ACR20/50/70 and DAS28-CRP response at 12 weeks, safety and tolerability, pharmacokinetics, and patient-reported outcomes.
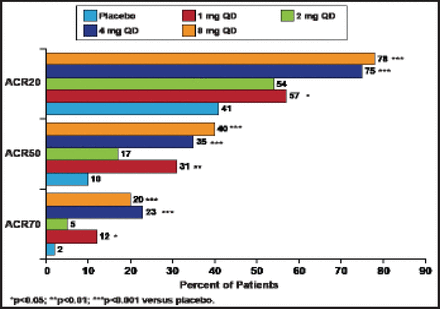
A total of 301 subjects (mostly women, mean disease duration of ∼5.5 years) were enrolled. Subjects in this study had active disease (tender joint count 22/68; swollen joint count 16/66; mean DAS28-CRP score of 5.5; mean Health Assessment Questionnaire [HAQ] score 1.2). At 12 weeks, 76% of subjects who received either 4 or 8 mg of baricitinib achieved the primary endpoint of ACR20 response compared with 41% of placebo-treated patients (p<0.001). Treatment with baricitinib also led to significant (p<0.05) improvements in ACR20/50/70 at all doses except 2 mg (Figure 1). DAS28-CRP <2.6 response was achieved by 37% of subjects in the 4-mg group and 22% of those in the 8-mg group (p<0.05 vs placebo). The onset of efficacy was rapid for ACR20/50/70 and DAS28-CRP, with statistically significant differences seen after 2 weeks of therapy. Simplified Disease Activity Index and Clinical Disease Activity Index remission was achieved more frequently by patients in the 4-mg group (18% and 22%, respectively; both p<0.05 vs placebo). Sixty percent of subjects in the 4-mg group and 67% of those in the 8-mg group achieved the minimum clinically important difference for the HAQ-Disability Index compared with placebo (41%) at Week 12.
ACR Responses by Dose at 12 Weeks.
Reproduced with permission from E. Keystone, MD. Keystone et al. Ann Rheum Dis 2012;71[Suppl3].
Adverse events (AEs) were mild, and no new safety signals were reported. Dose-dependent decreases in hemoglobin, small increases in serum creatinine, and increases in low- and high-density lipoprotein cholesterol were observed. Seven serious AEs were reported in 6 patients (2 in the placebo group, 3 in the 2-mg group, 1 in the 8-mg group). No deaths or opportunistic infections occurred in the active treatment groups. One patient in the placebo group was diagnosed with an opportunistic infection of toxocariasis. A similar rate of infection was observed in the placebo group (12%) and the combined treatment groups (14%), representing the most common treatment-emergent AE.
- © 2012 MD Conference Express®