Summary
Results from the Multi-center, Randomized, Blinded, Parallel-group Study of the Reduction of Signs and Symptoms During Monotherapy Treatment With Tocilizumab 8 mg/kg Intravenously Versus Adalimumab 40 mg Subcutaneously in Patients With Rheumatoid Arthritis trial [ADACTA; NCT01119859], suggest that tocilizumab monotherapy may be more effective than adalimumab monotherapy reducing the signs and symptoms of rheumatoid arthritis.
- Rheumatoid Arthritis Clinical Trials
Data from several registries and a United States health insurance claims database have shown that approximately one-third of rheumatoid arthritis (RA) patients are being treated with biologics as monotherapy [Yazici Y et al. Bull NYU Hosp Jt Dis 2008; Lee SJ et al. J Rheumatol 2009], but there have been no head-to-head studies to assist in the choice of monotherapy for these patients. Results from the Multi-center, Randomized, Blinded, Parallel-group Study of the Reduction of Signs and Symptoms During Monotherapy Treatment With Tocilizumab 8 mg/kg Intravenously Versus Adalimumab 40 mg Subcutaneously in Patients With Rheumatoid Arthritis trial [ADACTA; NCT01119859], presented by Cem Gabay, MD, University Hospitals, Geneva, Switzerland, suggest that tocilizumab monotherapy may be more effective than adalimumab monotherapy reducing the signs and symptoms of RA.
The ADACTA trial was an international, multicenter, randomized, double-blind, 24-week superiority trial that compared tocilizumab with adalimumab monotherapy in patients with RA. Patients were required to have an RA diagnosis of ≥6 months and a Disease Activity Score (DAS) score >5.1 and to be methotrexate-intolerant or judged inappropriate for continued treatment with methotrexate. Patients with prior treatment with a biologic agent were excluded. The primary study endpoint was mean change from baseline in the DAS28 at Week 24. Key secondary endpoints included efficacy at Week 24, based on the proportions of patients who achieved DAS28 remission (<2.6) and low disease activity (≤3.2), ACR 20/50/70 responses, and ACR/EULAR remission. Safety was assessed using adverse events (AEs) and laboratory parameters. Clinical Disease Activity Index (CDAI) responses were assessed as a post hoc analysis.
Study participants, aged 53 to 54 years and mainly women (82% in the adalimumab arm; 79% in the tocilizumab arm), had active disease (DAS 6.7 to 6.8; Health Assessment Questionnaire score 1.6 to 1.7) and a disease duration of 6.3 to 7.3 years. Subjects were randomized to intravenous tocilizumab 8 mg/kg every 4 weeks plus subcutaneous placebo (n=163) or adalimumab 40 mg subcutaneously every 2 weeks plus intravenous placebo (n=163). Patients who did not achieve at least a 20% improvement from baseline in swollen and tender joint count at Week 16 or later could escape to weekly subcutaneous injections of adalimumab/placebo.
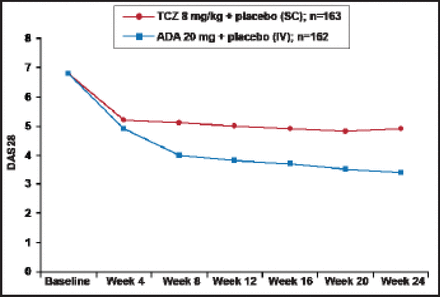
At Week 24, the change from baseline in DAS28 was −3.3 for the tocilizumab group versus −1.8 for subjects who received adalimumab (difference, 1.5; 95% CI for difference, −1.8 to −1.1; p<0.0001). Differences were observed, beginning at Week 8 (Figure 1). Significantly (p<0.0001) more subjects in the tocilizumab group also achieved the secondary endpoints of remission and low disease activity compared with those who received adalimumab (39.9% versus 10.5% and 51.5% versus 19.8%, respectively). ACR20/50/70 responses were also significantly (p<0.01) better among the tocilizumab subjects (65.0%, 47.2%, 32.5%) compared with subjects who received adalimumab (49.4%, 27.8%, 17.9%). In the post hoc analysis for CDAI response, 47.9% of tocilizumab versus 29.0% of adalimumab subjects (p=0.0003) were considered to be in remission or have low disease activity (CDAI score ≥0 to ≤10).
DAS28: Mean (±SE) Over Time.
TJC=tender joint count; SJC=swollen joint count; TCZ=tocilizumab; ADA=adalimumab; LOCF used for TJC and SJC; ESR and Patient's Global Assessment of Disease Activity VAS; If ESR=0 then ESR=1 is substituted into the DAS28 calculation to enable a non-missing DAS28.
Reproduced with permission from C. Gabay, MD.
The incidence of AEs was similar (82.1% in the tocilizumab arm and 82.7% in the adalimumab arm). Serious AEs and serious infections were also similar (tocilizumab: 11.7%, 3.1%; adalimumab: 9.9%, 3.1%). Changes in transaminase, low-density lipoprotein elevations, and neutrophil reductions occurred in both arms, with the proportion of patients with abnormal values higher in the tocilizumab arm. There were 2 deaths in the tocilizumab arm; 1 from sudden death that was considered to possibly be related to the study drug and 1 from illicit drug overdose that was not considered to be related.
- © 2012 MD Conference Express®