Summary
This article presents an overview of the Outcome Reduction With an Initial Glargine Intervention Trial [ORIGIN; NCT00069784]. ORIGIN was a large, international, randomized, controlled trial that lasted >6 years in people with new or recently diagnosed diabetes, impaired fasting glucose, impaired glucose tolerance, and additional cardiovascular (CV) risk factors. ORIGIN is the longest investigation of the effect of insulin treatment on CV outcomes and cancer incidence in this population to date.
- Diabetes Mellitus
- Featured Meeting - Specialty page
- Insulin
Gilles Dagenais, MD, Laval University Heart and Lung Institute, Quebec City, Quebec, Canada, and Ambady Ramachandran, MD, India Diabetes Research Foundation, Chennai, India, presented an overview of the Outcome Reduction With an Initial Glargine Intervention Trial [ORIGIN; NCT00069784].
ORIGIN was a large, international, randomized, controlled trial that lasted >6 years in people with new or recently diagnosed diabetes, impaired fasting glucose (IFG), impaired glucose tolerance (IGT), and additional cardiovascular (CV) risk factors.
ORIGIN is the longest investigation of the effect of insulin treatment on CV outcomes and cancer incidence in this population to date. The relationship between long-term supplementation with n-3 fatty acids and the rate of CV events was also studied [Gerstein H et al. N Engl J Med 2012].
ORIGIN Trial Design
ORIGIN was a double-blind study with a 2-by-2 factorial design. It was designed to test the effect of titrated basal insulin glargine versus standard care, and it also investigated n-3 fatty acid supplements versus placebo on CV outcomes [Gerstein H et al. Am Heart J 2008].
A total of 12,537 participants (mean age, 63.5 years; 35% women) were enrolled from 573 clinical sites in 40 countries. Patients were randomly assigned by region. The median follow-up was 6.2 years (interquartile range, 5.8 to 6.7 years). At the conclusion of the study, the primary outcome status was known for 99% of participants.
Coprimary outcomes in the glargine trial were the first occurrence of nonfatal myocardial infarction (MI) or nonfatal stroke or CV and the first occurrence of nonfatal MI or nonfatal stroke or CV death or hospitalization for heart failure or revascularization. Other outcomes and measures included new or recurrent cancers, angina, ischemia-related amputation, hypoglycemia, and CV and other hospitalizations.
n-3 Fatty Acid Trial Results
Jacqueline Bosch, MSc, McMaster University, Hamilton, Ontario, Canada, presented results from the n-3 fatty acid portion of the ORIGIN trial.
Baseline intake of n-3 fatty acids was 210 mg/day versus 209 mg/day for placebo. At the end of the study, it was 257 mg/day versus 253 mg/day for placebo. The primary outcome of the trial was CV death. Secondary outcomes were MI, stroke or CV death, all-cause mortality, presumed arrhythmic death, or cardiac arrest.
Patients were randomized to receive a daily supplement of n-3 fatty acid (1g per day) or placebo. The primary outcome of the trial was CV death. Secondary outcomes were MI, stroke or CV death, all cause mortality, presumed arrhythmic death, or cardiac arrest.
Omega-3 fatty acids did not reduce the rate of CV events in high-risk patients with diabetes or prediabetes. The rate of CV death was 9.1% in patients who were treated with placebo and 9.3% in patients who were treated with omega-3 fatty acids. Supplementation with n-3 fatty acids did not have a significant effect on major vascular events (16.5% versus 16.3%) or all-cause death (15.1% versus 15.4%), nor did it make a difference in fatal or nonfatal MI, fatal or nonfatal stroke, heart failure hospitalization, revascularization, limb or digit amputation, or hospitalization for any CV cause.
Except for a significant decrease of 14.5 mg/dL (0.16 mmol/L; p<0.001) in triglycerides in the group that took fish oil supplements, there was minimal difference in blood pressure, heart rate, and cholesterol.
Kaplan-Meier curves were virtually indistinguishable between arms for death from CV cause (HR, 0.98; 95% CI, 0.87 to 1.10; p=0.72); MI, stroke, or CV death (HR, 1.01; 95% CI, 0.93 to 1.10; p=0.81); all-cause death (HR, 0.98; 95% CI, 0.89 to 1.07; p=0.63); or death from arrhythmia (HR, 1.10; 95% CI, 0.93 to 1.30; p=0.26).
Previous randomized, double-blind, placebo-controlled trials have reported conflicting results on the efficacy of n-3 fatty acid supplements in the secondary prevention of CV disease.
Aldo P. Maggioni, MD, ANMCO Research Center, Florence, Italy, explained that these differences may be due to differences in background clinical conditions, risks, or therapies. In patients with dysglycemia and additional CV risk factors, 1 g of n-3 fatty acids daily over a period of 6 years does not reduce CV deaths or other CV outcomes.
The different risks of these patients and optimized background therapies may explain the neutral results of ORIGIN versus those of other trials that have been conducted in higher-risk populations. Additional large trials will provide important information that is related to n-3 fatty acids at various stages of CV disease. These include ASCEND [NCT00135226], the Rischio and Prevenzione study, and VITAL [NCT01169259].
Insulin Glargine Main Trial Results
Hertzel C. Gerstein, MD, MSc, McMaster University, Hamilton, Ontario, Canada, presented the main results from the insulin glargine trial.
The intervention (added to lifestyle change) for the insulin glargine group used the same approach for subjects with or without type 2 diabetes: add evening glargine to 0 or 1 oral agent; self-titrate at 1 to 2 units twice a week to target capillary fasting plasma glucose (FPG) ≤95 mg/dL (5.3 mmol/L); and add metformin if needed to mitigate hypoglycemia. The nondiabetes standard care group was screened for type 2 diabetes annually. Those with the disease received treatment, based on guidelines and physician's judgment.
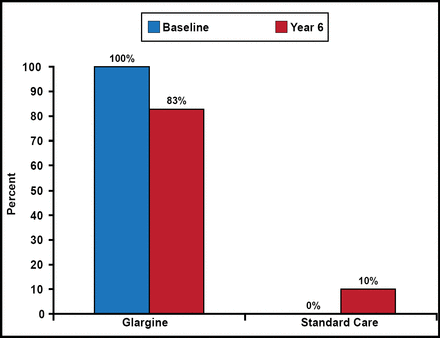
During the study, insulin use in the glargine group dropped from 100% to 83% at Year 6, while it rose from 0% to 10% in the standard care group (Figure 1). At the end of the study, median FPG in the glargine group was 95 mg/dL (5.3 mmol/L) versus 123 mg/dL (6.8 mmol/L) in the standard care group. At 6 years, median HbA1C levels were 6.2% for glargine and 6.5% for standard care.
Insulin Use.
Rates of incident CV outcomes were similar in the glargine and the standard care groups: 2.94 and 2.85 per 100 person-years, respectively, for the first coprimary outcome (MI, stroke, or CV death; HR, 1.02; 95% CI, 0.94 to 1.11; p=0.63) and 5.52 and 5.28 per 100 person-years, respectively, for the second coprimary outcome (MI, stroke, CV death, revascularization, heart failure; HR, 1.04; 95% CI, 0.97 to 1.11; p=0.27). Differences in the incidence of any cancer were not significant (HR, 1.00; 95% CI, 0.88 to 1.13; p=0.97).
Dr. Gerstein concluded that basal insulin glargine that is titrated to a normal FPG has a neutral effect on CV outcomes and on cancer compared with standard care.
Diabetes Prevention
Jeffrey L. Probstfield, MD, University of Washington School of Medicine, Seattle, Washington, USA, discussed diabetes prevention, asking the question: “Could insulin prevent diabetes?”
The predefined outcome of new diabetes that developed from the time of randomization up to and including an oral glucose tolerance test (OGTT) that was done approximately 1 month after all glucose-lowering therapies were stopped occurred in 24.7% versus 31.2% of 1456 participants without baseline diabetes (OR, 0.72; 95% CI, 0.58 to 0.91; p=0.006). A consistent but attenuated effect size was noted when the results of a second OGTT (done 3 months later in people without diabetes, based on the first OGTT) were included (OR, 0.80; 95% CI, 0.64 to 1.00; p=0.05) [Gerstein H et al. N Engl J Med 2012].
Dr. Probstfield concluded that although the durability of the effect is unclear, in people who are at risk for future diabetes, approximately 6 years of basal insulin glargine that is titrated to a normal FPG reduces the incidence of diabetes compared with standard care.
Safety: Hypoglycemia and Weight
Lars E. Rydén, MD, PhD, Karolinska University Hospital, Stockholm, Sweden, noted that insulin therapy has been known to cause hypoglycemia for 90 years and that it is also linked to CV outcomes. Whether or not the relationship is causal is controversial. To estimate benefits and risks of insulin glargine, ORIGIN sites collected information about severe and nonsevere episodes of clinical hypoglycemia at each visit.
Severe hypoglycemia was defined as: a) signs and/or symptoms of hypoglycemia; b) required assistance (unable to help self); and c) spontaneous recovery with carbohydrate/glucagon or any measured glucose ≤36 mg/dL (2 mmol/L). Nonsevere hypoglycemia was defined as signs and/or symptoms of hypoglycemia.
According to Dr. Rydén, there were significant differences between the two groups in all categories of hypoglycemia. Rates of severe hypoglycemia were 1.00 versus 0.31 per 100 person-years (p<0.001). Median weight increased by 1.6 kg (95% CI, 2.0 to 5.5; p<0.001) in the glargine group and decreased by 0.5 kg (95% CI, 4.3 to 3.2; p<0.001) in the standard care group during 6.2 years.
Implications for Insulin Therapy
Compared with standard glycemic care of people with early diabetes, IGT, and/or IFG, using once-daily basal insulin glargine to an FPG ≤95 mg/dL (5.3 mmol/L) for a median of 6.2 years maintains near-normal glycemic control; has a neutral effect on CV outcomes and on cancers; slows the progression of dysglycemia; and modestly increases hypoglycemia and weight.
The implications of these findings are that supplementing endogenous insulin with basal insulin injections slows progression of dysglycemia. Although later benefits or harms can not be ruled out, exogenous basal insulin's main effect over 6 to 7 years is to flexibly lower glucose.
Despite lower glucose levels, routine early use of basal insulin glargine is not better than guideline-based standard care in limiting important health outcomes. Basal insulin glargine is now the best-studied glucose-lowering drug that is available, and no new safety concerns limit its early use when needed.
The editors would like to thank the many members of the American Diabetes Association presenting faculty who generously gave their time to ensure the accuracy and quality of the articles in this publication.
- © 2012 MD Conference Express®