Summary
Incretins are hormones that stimulate insulin secretion in response to meals. Glucagon-like peptide-1 (GLP-1), an incretin secreted by enteroendocrine L-cells, stimulates insulin release, suppresses glucagon secretion, and reduces appetite, leading to the lowering of blood glucose. This article presents an overview of incretin-based therapies, and discussed the differences between the GLP-1RAs, as well as between dipeptidyl peptidase-4 inhibitors.
- Diabetes Mellitus
- Featured Meeting - Specialty page
- Hormone Therapy
Incretins are hormones that stimulate insulin secretion in response to meals. Glucagon-like peptide-1 (GLP-1), an incretin secreted by enteroendocrine L-cells, stimulates insulin release, suppresses glucagon secretion, and reduces appetite, leading to the lowering of blood glucose. Once in the circulation, GLP-1 has a half-life of less than 2 minutes because of rapid degradation by the enzyme dipeptidyl peptidase-4 (DPP-4), thus nullifying its antidiabetic properties. Two strategies have been employed to overcome this obstacle as a treatment for type 2 diabetes mellitus (T2DM). One is to use GLP-1 receptor agonists (GLP-1RAs), particularly those with a prolonged half-life, and the other is to inhibit the enzyme DPP-4, which prolongs the half-life of endogenously released active GLP-1 [Ahrén B, Schmitz O. Horm Metab Res 2004]. In an overview of incretin-based therapies, Filip K. Knop, MD, PhD, University of Copenhagen, Copenhagen, Denmark, discussed the differences between the GLP-1RAs. Adrian Vella, MD, Mayo Clinic, Rochester, Minnesota, USA, discussed differences between DPP-4 inhibitors.
Prof. Knop compared the pharmacokinetics, immunogenicity, and molecular sizes of 5 GLP-1RAs: exenatide BID, liraglutide, and exenatide QW, which are currently on the market, plus lixisenatide and albiglutide, which are in clinical trials. He compared these agents in terms of pharmacokinetics (short-acting versus continuous-acting), structure (exendin-4-based versus GLP-1-based) and size (small versus large molecule). Exenatide BID and lixisenatide are considered short-acting agonists, while exenatide QW, liraglutide, and albiglutide are continuous-acting (Table 1). GLP-1 lowers postprandial glycemia, primarily through inhibitory effects on gastric emptying, in healthy subjects and in those with T2DM [Nauck MA et al. Diabetes 2011]. Short-acting GLP-1RAs better preserve GLP-1-induced reduction in gastric emptying and postprandial glucose control compared with long-acting agonists; however, nausea and vomiting appear to be greater with short-acting versus long-acting agonists [Rosenstock J et al. Diabetes Care 2009]. Nevertheless, continuous-acting GLP-1RAs seem to have a greater effect on glycemic control (HbA1C), most likely because of their superior effect on fasting plasma glucose (FPG).
Comparison of GLP-1 Receptor Agonists.
Antibody formation in response to therapeutic peptides is common. Only 9% of patients who were treated with the GLP-1-based liraglutide developed antibodies toward the drug, whereas treatment with the GLP-1RAs that were based on exendin-4 elicited antibodies in about 50% of patients. Although it is controversial whether antibody formation is associated with reduced efficacy, patients who develop antibodies tend to have more injection-site reactions [Fineman MS et al. Diabetes Obes Metab 2012].
GLP-1-receptors are present in several central nervous system (CNS) regions. When activated, they are believed to be involved in inducing the feeling of satiety, which in turn, results in decreased food intake and weight loss. The small GLP-1RAs liraglutide and exenatide are capable of directly penetrating the blood-brain barrier and activating CNS centers in animals [Baumgartner et al. J Neuroendocrinol 2010], while albiglutide, with its linkage to human albumin, is considered too large to pass through the small fenestras in the blood-brain barrier. The reduced efficacy of these compounds compared with the small GLP-1RAs may be associated with less satiety and perhaps fewer side effects, such as nausea and vomiting.
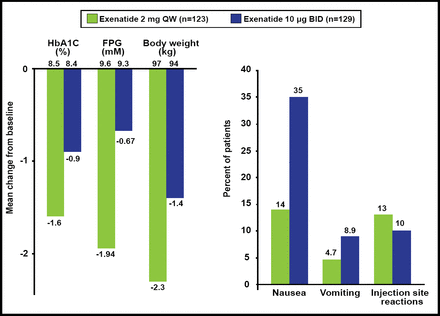
All of these differences explain the results of the head-to-head comparisons that have been made with GLP-1RAs. In the Diabetes Therapy Utilization: Researching Changes in A1C, Weight and Other Factors Through Intervention with Exenatide Once Weekly [DURATION-1; Drucker DJ et al. Lancet 2008] and −5 [Blevins T et al. J Clin Endocrinol Metab 2011] studies, the short-acting agent (exenatide BID) had a better effect on postprandial glucose but was associated with more nausea and vomiting, whereas the long-acting agent (exenatide QW) elicited the most pronounced effect on fasting plasma glucose (Figure 1). In the Effect of Liraglutide or Exenatide Added to an Ongoing Treatment on Blood Glucose Control in Subjects With Type 2 Diabetes [LEAD-6; NCT00518882] study, significantly more patients developed antibodies when treated with GLP-1-based (liraglutide) versus exendin-4-based (exenatide) molecules [Buse JB et al. J Clin Endocrinol Metab 2011]. There was some suggestion that high levels of anti-exenatide antibodies were correlated with smaller HbA1C reductions.
Main 24-Week Results: DURATION-5.
Reproduced with permission from LF Meneghini, MD.
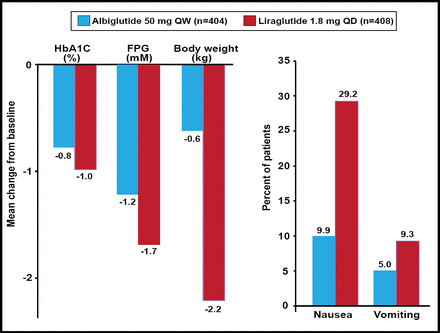
In the Study to Determine the Efficacy and Safety of Albiglutide as Compared With Liraglutide [HARMONY-7; NCT01128894] study, GLP-1-based albiglutide, a long-acting large molecule, was compared with liraglutide, a long-acting small molecule that is also based on GLP-1. Significant reductions in FPG and HbA1C were observed with both treatments, although less so with albiglutide. Compatible with the idea that albiglutide is too large to pass through the blood-brain barrier as efficiently as the much smaller liraglutide moiety, significantly less nausea and vomiting were noted with albiglutide (Figure 2) [Pratley et al. ADA 2012 Abstract 945P]. Thus, Prof. Knop concluded that GLP-1 receptor agonists are not the same. More work is needed to tease out these differences.
Top-Line 32-Week Results: HARMONY-7.
Reproduced with permission from LF Meneghini, MD.
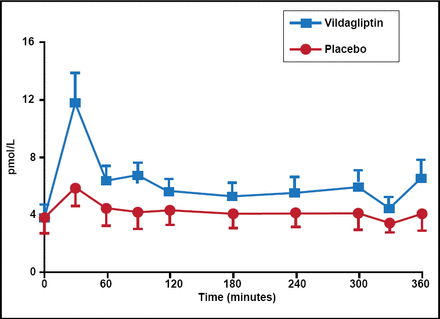
DPP-4 inhibitors enhance glucose-dependent insulin secretion from pancreatic β-cells by preventing DPP-4-mediated degradation of endogenously released GLP-1, but not by altered insulin action, effects on gastric emptying, or rate of entry of ingested glucose into the systemic circulation (Figure 3) [Dalla Man C et al. Diabetes Care 2009; Vella A et al. Diabetes 2007]. These inhibitors represent a new therapeutic approach to the management of T2DM. Dr. Vella included 5 DPP-4 inhibitors in his overview: vildagliptin, sitagliptin, saxagliptin, alogliptin, and linagliptin. The DPP-4 inhibitors have important pharmacokinetic differences, including half-life, systemic exposure, bioavailability, protein binding, metabolism, presence of active metabolites, and excretion routes. The latter is particularly relevant in patients with renal or hepatic impairment.
DPP-4 Inhibitors Alter Concentrations of GLP-1.
Reproduced with permission from the American Diabetes Association. Vella A et al. Effects of Dipeptidyl Peptidase-4 Inhibition on Gastrointestinal Function, Meal Appearance, and Glucose Metabolism in Type 2 Diabetes. Diabetes. May 2007;56(5):1475–1480.
With the exception of vildagliptin (half-life=2 to 3 hours), a single daily dose of any of these inhibitors provides 24-hour inhibition of DPP-4. They have a large apparent volume of distribution, bind poorly to plasma proteins (with the exception of linagliptin), are not metabolized by cytochrome P450, and the metabolites have little activity (with the exception of saxagliptin). The pharmacokinetics of linagliptin is different from that of the others in that it is excreted (85%) in the feces. DPP-4 inhibitors also have off-target mechanisms, such as the ability to physiologically cleave cytokines, chemokines, and neuropeptides that are involved in inflammation, immunity, and vascular function, and, thus, it is thought that they hold promise for cardiovascular protection. One study has shown that sitagliptin increases circulating vasculoprotective endothelial progenitor cells in patients with T2DM, with concomitant upregulation of stromal-derived factor-1α. This ancillary effect of DPP-4 inhibition might have potentially favorable cardiovascular implications [Fadini GP et al. Diabetes Care 2010].
Several Phase 3 trials of DPP-4 inhibitors are ongoing and may shed light on the long-term efficacy and safety of DPP-4 inhibitor therapy, the effect on pancreatic cell function and peripheral glucose metabolism, and the effect on cardiovascular outcomes in patients with T2DM. To date, the data indicate that DPP-4 inhibitors have similar glucose-lowering efficacy, a similar weight-neutral effect, and comparable safety and tolerability profiles.
- © 2012 MD Conference Express®