Summary
The basal insulin analog LY2605541 (LY) is a long-acting, PEGylated insulin lispro that is designed with a large hydrodynamic size, which delays insulin absorption and reduces clearance, resulting in prolonged duration of action. LY demonstrates a preferential hepatic effect compared with human insulin in a somatostatin-infused/glucagon-replaced conscious dog model [Moore MC et al. ADA 2012 Abstract 1609P]. This article discusses data that showed that in a continuously glucose-monitored cohort of patients with type 2 diabetes mellitus, those receiving LY had lower intra-day glucose variability and fewer and shorter episodes of hypoglycemia compared with patients receiving glargine.
- Diabetes Mellitus
- Insulin
- Hyperglycemia/Hypoglycemia
The basal insulin analog LY2605541 (LY) is a long-acting, PEGylated insulin lispro that is designed with a large hydrodynamic size, which delays insulin absorption and reduces clearance, resulting in prolonged duration of action. LY demonstrates a preferential hepatic effect compared with human insulin in a somatostatin-infused/glucagon-replaced conscious dog model [Moore MC et al. ADA 2012 Abstract 1609P]. Richard Bergenstal, MD, International Diabetes Center, Park Nicollet, Minneapolis, Minnesota, USA, presented data that showed that in a continuously glucose-monitored cohort of patients with type 2 diabetes mellitus (T2DM), those receiving LY had lower intra-day glucose variability and fewer and shorter episodes of hypoglycemia compared with patients receiving glargine.
Hypoglycemia and glucose variability were assessed with continuous glucose monitoring (CGM) of interstitial glucose (IG) in a subset of patients from a Phase 2, randomized, open-label, parallel study of T2DM patients who were administered LY (n=51) or insulin glargine (GL; n=25). CGM was conducted on 3 consecutive days (72 to 84 hours) during the week before Week 0, 6, and 12 study visits. A hypoglycemic episode was defined as IG ≤70 mg/dL and continued until IG was >70 mg/dL for 15 minutes (or 3 time points).
At 12 weeks, LY-treated patients spent less time with IG <70 mg/dL than GL-treated patients during the 24-hour period (25±6 versus 83±16 min; p=0.01) and during the nocturnal period (11±5 versus 38±13 min; p=0.024). Significantly fewer LY- than GL-treated patients experienced hypoglycemia (50.0% versus 78.3%; p=0.036), including nocturnal hypoglycemia (20.5% versus 47.8%; p=0.027) [IMAGINE 1–5; NCT01481779; NCT01435616; NCT01454284; NCT01468987; NCT01582451]. Further CGM evaluation is ongoing in Phase 3 studies of LY2605541 in patients with type 1 diabetes mellitus (T1DM) and T2DM.
Insulin degludec (IDeg) is a new-generation, ultralong-acting basal insulin, offered in 100-U/mL and 200-U/mL doses. Previous T1DM studies have identified the IDeg 100-U/mL dose as having a flat and stable action profile, with the ability to control glucose beyond 42 hours. Steady-state is reached within 2 to 3 days, and terminal half-life is twice (25.4 hrs) as long as that of insulin glargine (12.5 hrs) [Heise T et al. Diabetologia 2011; Heise T et al. ADA 2012 Abstract 1013P].
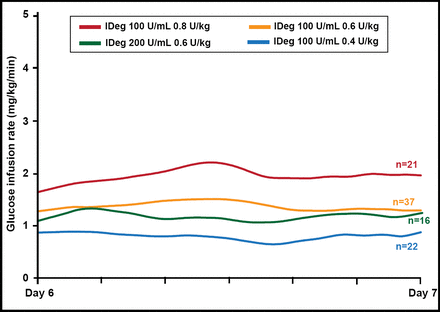
Tim Heise, MD, Profil Institut für Stoffwechselforschung, Neuss, Germany, presented pharmacodynamic and pharmacokinetic data for the 200-U/mL (0.6 U/kg once daily over 6 days) dose of IDeg in T2DM patients (n=16; mean body mass index (BMI), 30 kg/m2; HbA1C, 7.3%; age, 59.4 years). On Day 6, while at steady state, a 26-hour euglycemic glucose clamp was conducted (Biostator; clamp blood glucose level: 90 mg/dL). The mean glucose infusion rate for the IDeg 200-U dose was flat and stable over the dosing interval and similar to the 100-U dose (Figure 1). The glucose-lowering effect of IDeg was evenly distributed over the 24-hour dosing interval. The effect of IDeg extended beyond 26 hours in all subjects. The terminal half-life at steady state was 25.1 hours. IDeg 200-U/mL was well tolerated and safe, with no injection site reactions. IDeg 200-U/mL has a flat, stable glucose-lowering effect and ultralong duration of action, with pharmacodynamic and pharmacokinetic characteristics that are similar to the 100-U/mL dose.
IDeg: Glucose-Lowering Effect at Steady State.
Reproduced with permission from T Heise, MD.
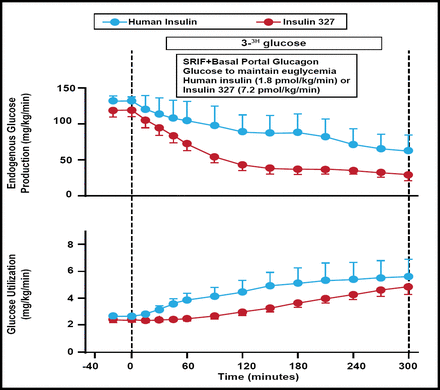
Hepatopreferential insulin analogs functionally restore the physiological insulin gradient that is lost when insulin is delivered subcutaneously. Normalizing the distribution of insulin's effects at the liver versus nonhepatic tissues may reduce glycemic fluctuations, correct dyslipidemia, decrease vascular disease, reduce weight, limit hypoglycemic risk, and increase hepatic glucose uptake and glycogen storage. Dale S. Edgerton, PhD, Vanderbilt University Medical Center, Nashville, Tennessee, USA, presented results from an animal study that showed the peripheral delivery of a novel hepatopreferential insulin analog (insulin 327) leads to delayed suppression of lipolysis, greater effect on liver glucose metabolism, and reduced effect on nonhepatic glucose uptake compared with regular insulin delivered by the same route. Dogs with arterial, portal, and hepatic vein catheters were studied after an 18-hour fast. 3H-glucose was infused from −140 min. After a basal period (−40 to 0 min), somatostatin and basal portal glucagon were infused (0 to 300 min). At the same time, insulin 327 or human insulin (HI) was infused into a peripheral vein (7.2 or 1.8 pmol/kg/min, respectively; n=5/group), and euglycemia was maintained by glucose infusion. Suppression of lipolysis was delayed with insulin 327 compared with HI: during the first hour, plasma nonesterified fatty acid levels (µmol/L) decreased by 91±37 versus 419±42, respectively, and blood glycerol levels (µmol/L) fell by 0±7 versus 39±5, respectively. Arterial insulin 327 and HI levels increased to 10,870±2543 and 95±8 pmol/L, respectively (Figure 2).
Glucose Appearance and Utilization.
Reproduced with permission from DS Edgerton, PhD.
Relative to the basal period, insulin 327 suppressed net hepatic glucose balance and glucose production (mg/kg/min; last 3 h) by 1.9±0.1 and 1.7±0.1, respectively, while HI reduced them by only 0.5±0.6 and 1.1±0.3. On the other hand, insulin 327 increased nonhepatic glucose uptake and glucose utilization (mg/kg/min; last 3 h) by only 1.0±0.4 and 1.5±0.3, respectively, while HI increased them by 2.4±1.2 and 2.5±1.1. Liver-preferential insulin analogs may provide a therapeutic benefit by functionally restoring the physiological insulin gradient at the liver compared with peripheral tissues.
Recombinant human hyaluronidase (rHuPH20) is a genetically engineered soluble version of the naturally occurring human hyaluronidase enzyme that is used to accelerate absorption and action of insulin and, thus, improve prandial glucose control. It has a history of more than 60 years of safe clinical use. The aim of this study was to compare rapid-acting analog insulin that is formulated with rHuPH20 versus insulin lispro alone on glycemic control parameters, safety, and tolerability in an intensive basal-bolus insulin therapy in patients with type 1 diabetes [Phase II Study Evaluating Pharmacokinetics and Postprandial Glycemic Response of Subcutaneously Injected Humalog and Humlin R With/Without Co Injected Recombinant Human Hyaluronidase Following Liquid Meal in Type1 Diabetes Mellitus Patients; NCT00774800]. The results were presented by Irl B. Hirsch, MD, University of Washington School of Medicine, Seattle, Washington, USA.
After a 4- to 6-week run-in using prandial glulisine plus glargine (BID), 117 subjects (mean: age 42.6±14 years; BMI, 27.3±4.4 kg/m2; HbA1C, 6.7% to 8.3%) were randomly assigned (double-blind crossover) to lispro plus rHuPH20 or aspart plus rHuPH20 versus lispro alone for 2 12-week intensive management periods; prandial doses were given immediately before meals. The primary endpoint of HbA1C noninferiority (0.4% margin) was achieved with no treatment difference (95% CI, −0.05 to 0.15). Overall hypoglycemic rates (≤70 mg/dL or symptoms) were reduced 5% (p=0.035), and events <56 mg/dL were reduced 7% (p=0.045). Total daily insulin dose (54±27 for analog-PH20 versus 56±27 U for lispro; p=0.057) and weight gain difference (−0.57 lb; p=0.27) showed favorable trends. No meaningful differences in adverse events, immunogenicity, or injection-site pain were noted. Flatter daytime glucose profiles and an 82% reduction in postprandial glucose excursions suggest that analog-PH20 offers improved postprandial glucose control.
- © 2012 MD Conference Express®