Summary
In the Phase 3 Aflibercept Versus Placebo in Combination With Irinotecan and 5-FU in the Treatment of Patients With Metastatic Colorectal Cancer After Failure of an Oxaliplatin Based Regimen [VELOUR; NCT00561470] trial, aflibercept plus FOLFIRI improved overall survival (OS) compared with FOLFIRI plus placebo in patients with metastatic colorectal cancer. This subgroup analysis of the VELOUR trial evaluated the consistency of aflibercept's effect on OS and progression-free survival in a prespecified analysis of patients previously treated with bevacizumab.
- gastrointestinal cancers clinical trials
Bevacizumab (BEV) is a standard component of frontline therapy and FOLFIRI remains a standard chemotherapy backbone for second-line treatment of metastatic colorectal cancer (mCRC). Aflibercept is a recombinant human fusion protein that acts as a decoy receptor, preventing the interaction of vascular endothelial growth factor (VEGF)-A, VEGF-B, and placental growth factor (PlGF) with their receptors. In the Phase 3 Aflibercept Versus Placebo in Combination With Irinotecan and 5-FU in the Treatment of Patients With Metastatic Colorectal Cancer After Failure of an Oxaliplatin Based Regimen [VELOUR; NCT00561470] trial, aflibercept plus FOLFIRI improved overall survival (OS) compared with FOLFIRI plus placebo in patients with mCRC. This subgroup analysis of the VELOUR trial, presented by Carmen Joseph Allegra, MD, University of Florida, Gainesville, Florida, USA, evaluated the consistency of aflibercept's effect on OS and progression-free survival (PFS) in a prespecified analysis of patients previously treated with BEV.
In the VELOUR study, patients with mCRC were randomly assigned to aflibercept plus FOLFIRI (n=600) or placebo plus FOLFIRI (n=600). The primary endpoint was OS. Patients were allowed only one prior oxaliplatin-containing regimen for metastatic disease. Patients who relapsed within 6 months of completion of oxaliplatin-based adjuvant chemotherapy were eligible. The overall results showed that adding aflibercept to FOLFIRI in mCRC patients previously treated with oxaliplatin-based therapy significantly improved OS and PFS.
For the prespecified subgroup analysis, a p value of <0.1 would indicate a difference in the benefit associated with aflibercept between the prior and no prior BEV groups. Among patients with prior BEV therapy, 186 received aflibercept plus FOLFIRI and 187 received placebo plus FOLFIRI. Among patients with no prior BEV, 426 received aflibercept plus FOLFIRI and 427 received placebo plus FOLFIRI.
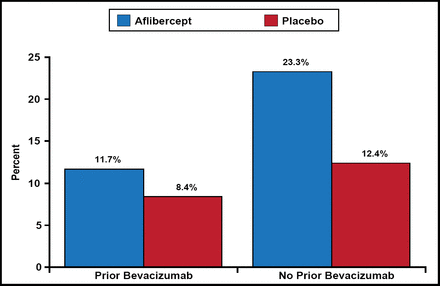
The OS and PFS results were consistent with and without prior bevacizumab. The interaction between the “treatment arm” and “prior bevacizumab” factor was not significant at the two-sided 10% level (p=0.57 for OS; p=0.20 for PFS; Table 1). Among patients with prior bevacizumab, those who received aflibercept had a median OS of 12.5 months versus 11.7 months in patients who received placebo (HR, 0.862; 95.34% CI; 0.673 to 1.104). Among patients with no prior bevacizumab, those treated with aflibercept had a median OS of 13.9 months versus 12.4 months in patients treated with placebo (HR, 0.788; 95.34% CI, 0.699 to 0.927). Response rates in the prior bevacizumab patients were 11.7% in the aflibercept arm versus 8.4% in the placebo arm. Response rates in patients without prior bevacizumab were 23.3% in the aflibercept arm versus 12.4% in the placebo arm (Figure 1).
Consistency of OS and PFS With and Without Prior BEV.
Response Rates.
The safety analysis showed increased anti-VEGF-associated events and general adverse events in the aflibercept arms but no difference between the prior and no prior BEV groups. Rates of adverse events leading to discontinuation were higher in the aflibercept arms, but there was no difference between the prior and no prior BEV groups.
This preplanned subgroup analysis demonstrates consistent trends of increased OS and PFS with aflibercept, regardless of prior treatment with BEV. Prior treatment with BEV does not appear to affect the safety profile of aflibercept. Although analysis of a prespecified subgroup, this study was not powered to show a treatment difference between arms; therefore, no definitive conclusions can be drawn concerning the benefit of aflibercept in the prior BEV-treated subgroup.
- © 2012 MD Conference Express