Summary
This article delves into the early gains that were made in the treatment of AMI, noting that the field has changed dramatically since 1912, when James B. Herrick, MD, postulated that thrombosis in the coronary artery leads to the symptoms and abnormalities that are associated with heart attacks [Herrick JB. JAMA 1912]. It further covers the major advances over the subsequent 100 years and provided a vision for cardiovascular medicine in the coming century.
- Myocardial Infarction Genomics
Cardiovascular leader and innovator Eugene Braunwald, MD, MACC, Harvard Medical School, Boston, Massachusetts, USA, launched The Legends of Cardiovascular Medicine series by delivering the 2012 Simon Dack Lecture, which focused on the treatment of acute myocardial infarction (AMI) – into the second century after Herrick.
The lecture delved into the early gains that were made in the treatment of AMI, noting that the field has changed dramatically since 1912, when James B. Herrick, MD, postulated that thrombosis in the coronary artery leads to the symptoms and abnormalities that are associated with heart attacks [Herrick JB. JAMA 1912]. Dr. Braunwald covered the major advances over the subsequent 100 years and provided a vision for cardiovascular medicine in the coming century.
Myocardial Infarction: The Early Days
Early pioneers in cardiovascular medicine were faced with a highly lethal disease, as patients with myocardial infarction (MI) experienced a 30% in–hospital mortality rate or frequently died before they could get to a hospital. The first major therapeutic advance occurred in 1961, with Dr. Desmond Julian's creation of the coronary care unit [Julian DG. Lancet 1961] which segregated patients with AMI, carefully monitored them with alarms, and performed closed chest resuscitation of previously fatal arrhythmias. This development reduced the mortality rate by half.
Other breakthroughs have included reperfusion therapy, which has been associated with a 75% decrease in mortality over a 25–year period; the addition of aspirin therapy to the MI treatment regimen; balloon angioplasty; and the use of both bare–metal and drug–eluting stents. These advances have continued to make impressive reductions in the mortality rate, but MI still exacts a high toll in morbidity and mortality in the United States (US) and around the world.
A Relentless Foe
Even with these impressive gains, MI remains a major health concern, with almost 1 million new cases a year in the US alone. An American citizen experiences an MI approximately every 34 seconds, and the attack will be fatal about 15% of the time [Roger VL et al. Circulation 2012].
The estimated annual incidence of MI among Americans is 610,000 new attacks and 325,000 recurrent ones; the annual mortality rate is 134,000. Worldwide, the figures are even more staggering. Every year, 17.1 million lives—82% of which are in the developing world—are claimed by the global burden of cardiovascular disease (CVD) [World Heart Federation. State of the Heart Cardiovascular Disease Report 2012].
Therapeutic Approaches to MI
Three important targets for therapy in AMI are the prevention of lethal myocardial reperfusion injury, post–MI inhibition of thrombin generation, and post–AMI cell therapy.
In a 1985 article in the Journal Clinical Investigation, Braunwald and Kloner described the double–edged nature of reperfusion—how it can limit infarct size but also lead to irreversible myocardial damage or death. The challenge of this dichotomy has led researchers to investigate options such as ischemic preconditioning [Murry CE et al. Circulation 1986], remote ischemic preconditioning [Przyklenk K et al. Circulation 1993], and perconditioning [Schmidt MR et al. Am J Physiol Heart Circ 2007; Botker HE. Lancet 2010]. A small proof–of–concept trial by Piot et al. [N Engl J Med 2008] found that the use of cyclosporin A was associated with approximately 40% smaller infarcts, presumably by inhibiting the opening of mitochondrial permeability transition pores.
Another area of great promise is the inhibition of thrombin generation post–MI, which can persist for months after an acute event. The ATLAS ACS 2–TIMI 51 trial [Mega JL et al. N Engl J Med 2011; Vinall M. MD Conference Express: AHA 2011] randomized 15,526 patients to one of two doses of rivaroxaban (2.5 mg or 5 mg) or placebo for a mean of 13 months and up to 31 months. Rivaroxaban significantly reduced the primary efficacy endpoint, compared with placebo (8.9% vs 10.7%; HR, 0.84; p=0.008 mITT; p=0.002 ITT). The 2.5–mg BID dose reduced the rate of death from cardiovascular causes (2.7% vs 4.1%; p=0.002) and from any cause (2.9% vs 4.5%; p=0.002). The primary safety endpoint of non–CABG TIMI major bleeding was increased with rivaroxaban (2.1% vs 0.6%; HR, 3.96; p<0.001), with no excess of fatal intracranial hemorrhage or fatal bleeding with rivaroxaban 2.5 mg BID and no evidence of hepatotoxicity or posttreatment rebound ischemic events. The trial was a landmark study that culminated a 2–decade search for another approach to combining antiplatelet and anticoagulant therapies.
The Next Frontiers
Several active areas of investigation hold promise for future advances in cardiovascular science and medicine, including genetics and genomics, molecular targeting, pharmacogenomics, and stem cell biology and regenerative medicine [Nabel EG, Braunwald E. N Engl J Med 2012].
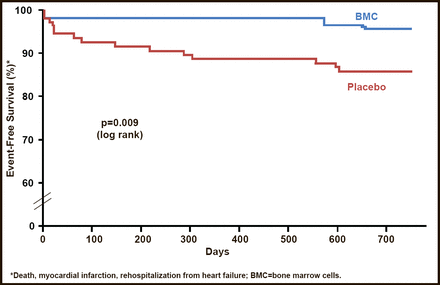
Post–AMI cell therapy, in particular the REPAIR–AMI trial [Assmus B et al. Circ Heart Fail 2010], showed that intracoronary infusion of bone marrow–derived mononuclear cells in patients with reperfused AMI is associated with improved global contractile function; preferentially improves left ventricular function in patients with the most severely depressed contractility after AMI; and prevents left ventricular end–systolic volume expansion within 4 months of therapy. The therapy holds great promise in limiting the development of postinfarction heart failure (Figure 1).
Two–Year Event–Free Survival: REPAIR–AMI.
Assmus B et al, Clinical Outcome 2 Years After Intracoronary Administration of Bone Marrow–Derived Progenitor Cells in Acute Myocardial Infarction, Circulation Heart Failure 2010, volume 3, issue number 1, page 89.
This lecture was inspiring both in recounting the tremendous progress that has been made in limiting the morbidity and mortality associated with AMI, and in highlighting the exciting innovations that hold promise in further reducing the global burden of AMI and its associated complications.
The editors would like to thank the many members of the American College of Cardiology presenting faculty who generously gave their time to ensure the accuracy and quality of the articles in this publication.
- © 2012 MD Conference Express®