Summary
Type 2 diabetes mellitus (T2DM) is a major risk factor for ischemic heart disease, and cardiovascular disease (CVD) is the leading cause of morbidity and mortality for individuals with T2DM [McEwen LN et al. Diabetes Care 2012]. Common conditions that coexist with T2DM (eg, hypertension and dyslipidemia) are clear risk factors for CVD; however, a diagnosis of T2DM itself confers independent risk [Whittington HJ et al. Cardiol Res Pract 2012].
- Cardiometabolic Disorder
- Diabetes Mellitus
Type 2 diabetes mellitus (T2DM) is a major risk factor for ischemic heart disease, and cardiovascular disease (CVD) is the leading cause of morbidity and mortality for individuals with T2DM [McEwen LN et al. Diabetes Care 2012]. CVD is also the largest contributor to direct and indirect medical costs that are associated with T2DM. Common conditions that coexist with T2DM (eg, hypertension and dyslipidemia) are clear risk factors for CVD; however, a diagnosis of T2DM itself confers independent risk [Whittington HJ et al. Cardiol Res Pract 2012].
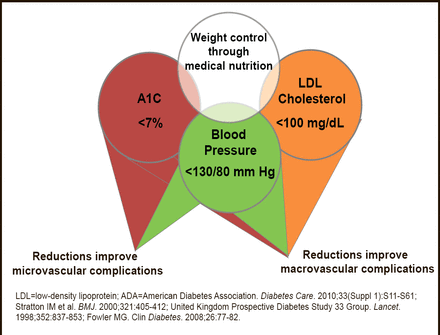
Numerous studies have demonstrated the efficacy of targeting and controlling individual CV risk factors (eg, blood pressure less than 130/80 mm Hg, low–density lipoprotein cholesterol less than 100 mg/dL, HbA1C <7%) in preventing or slowing the progression of microvascular and macrovascular disease in patients with T2DM [American Diabetes Association Standards of Medical Care in Diabetes—2012. Diabetes Care 2012] (Figure 1). Larger benefits are seen when multiple risk factors are globally addressed in patients with T2DM [Buse JB et al. Diabetes Care 2007; Gaede P et al. N Engl J Med 2008].
Metabolic Components of Diabetes: ADA Treatment Recommendations.
Reproduced with permission from EA Oral, MD.
However, randomized clinical trials have also suggested the limits of intensive CV risk factor control in T2DM [The ACCORD Study Group. N Engl J Med 2010; Duckworth W et al. N Engl J Med 2009; ADVANCE Collaborative Group. N Engl J Med 2008]. In particular, achieving intensive glucose control alone may be insufficient to reduce major CVD events. A new medication class that may reduce CVD in patients with T2DM uses molecules that activate the incretin system to raise or mimic glucagon–like peptide–1 (GLP–1). In a recent review, Motta et al. [Recent Pat Cardiovasc Drug Discov 2012] reported that incretin–based agents improve glycemic control by mechanisms that minimize hypoglycemia and that some agents also improve lipoprotein profiles, blood pressure control, and weight loss.
GLP–1 receptors have been discovered on cardiac myocytes and endothelial cells [Ban K et al. Circulation 2008; Bose AK. Diabetes 2005], and intravenous GLP–1 acutely improves left ventricular ejection function (LVEF) and reduces BNP levels in heart failure patients [Sokos GG et al. Card Fail 2006]. A 72–hour GLP–1 infusion also improved left ventricular wall motion abnormalities and LVEF in patients with a history of myocardial infarction (MI) [Nikolaidis LA et al. Circulation 2004]. Given all of these favorable effects on surrogate outcomes, there are currently large ongoing trials of GLP–1 agonists in patients with T2DM that are studying their ability to reduce CV endpoints [LEADER, NCT01179048; EXSCEL, NCT01144338].
Dipeptidyl peptidase 4 (DPP–4) inhibitors are another form of incretin–based therapy that indirectly increase endogenous GLP–1. Evidence shows that GLP–1 receptor agonists and DPP–4 inhibitors are capable of preserving myocardial function and protecting cardiac myocytes from ischemic damage, independent of their glucose–lowering function [Mannucci E, Dicembrini I. Curr Med Res Opin 2012].
Mannucci and Dicembrini note that both classes of drugs enhance endothelial function. In addition, DPP–4 inhibitors increase the availability of endothelial progenitor cells via a GLP–1 receptor–independent pathway. Taken together, available experimental evidence, with a few pilot studies in humans, suggests that incretin–based therapies could prevent CVD [Monami M et al. Exp Diabetes Res 2011; Phung OJ et al. JAMA 2010; Frederich R et al. Postgrad Med 2010]. As a result, there are several large randomized clinical trials studying the effects of DPP–4 inhibitors in patients with T2DM to reduce incident and recurrent CV events [TECOS, NCT00790205; EXAMINE, NCT00968708; SAVOR–TIMI 53, NCT01107886].
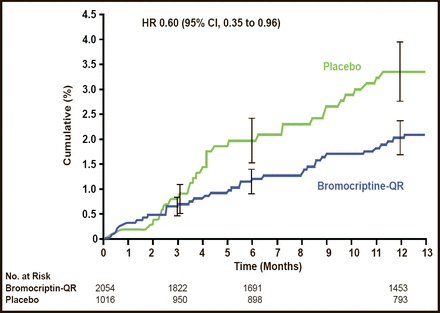
Neuroendocrine–based therapies are another approach of interest for reducing CVD in patients with T2DM. Quick–release bromocriptine (bromocriptine–QR) is a D2 dopamine receptor agonist. The Cycloset Safety Trial, a 52–week, randomized, double–blind, multicenter trial demonstrated the potential CV safety and efficacy of this novel therapy for T2DM (Figure 2) [Gaziano JM et al. Diabetes Care 2010]. Fewer people reported a CVD end point (the composite of MI, stroke, coronary revascularization, and hospitalization for angina or congestive heart failure) in the bromocriptine–QR group (1.8%) versus placebo group (3.2%; HR, 0.60; 95% two–sided CI, 0.35 to 0.96; Figure 2). The frequency of serious adverse events (SAEs) was comparable between the groups (8.6% vs 9.6%; HR, 1.02; 96% one–sided CI, 1.27).
CV Endpoints – Reported SAEs.
Reproduced from Gaziano JM et al. Randomized clinical trial of quick–release bromocriptine among patients with type 2 diabetes on overall safety and cardiovascular outcomes. Diabetes Care 2010. Jul;33(7):1503–8. With permission from the American Diabetes Association.
Incretin– and neuroendocrine–based therapies for patients with T2DM are exciting new developments; with the potential to improve overall CVD risk based on experimental and early clinical data. Although these early developments are promising, we await the results of the ongoing large multicenter clinical trials that are designed to determine whether these therapies reduce CV events in patients with T2DM.
- © 2012 MD Conference Express®