Summary
Effective adjunctive treatment of acute coronary syndrome with antithrombin therapy is essential to reduce ischemic complications, and researchers continue to compare novel antithrombotic agents with currently available ones in an effort to optimize outcomes. These comparisons require careful evaluation of the benefit-risk balance, with a primary consideration of reduction of ischemic complications weighed against bleeding risk.
- Thrombotic Disorders
- Myocardial Infarction
Effective adjunctive treatment of acute coronary syndrome (ACS) with antithrombin therapy is essential to reduce ischemic complications, and researchers continue to compare novel antithrombotic agents with currently available ones in an effort to optimize outcomes. These comparisons require careful evaluation of the benefit–risk balance, with a primary consideration of reduction of ischemic complications weighed against bleeding risk.
Unfractionated heparin (UFH) was once the standard of care for patients with ACS based on studies that showed that the combination of aspirin and heparin was associated with a substantially lower risk of myocardial infarction (MI) and death than either agent alone or placebo for patients with unstable angina or non–ST–elevation MI (NSTEMI) [Wallentin L et al. Lancet 1990; Oler A et al. JAMA 1996].
However, a primary problem with UFH is the variability in its therapeutic effect, said Marc Cohen, MD, FACC, Newark Beth Israel Medical Center, New Jersey, USA. In one study, the initial activated partial thromboplastin times (aPTTs; measured 4 to 8 hours after the start of therapy) were therapeutic in one–third of patients, were low in approximately 20%, were high in approximately 17%, and were markedly low and markedly high in 13% and 16%, respectively [Cheng S et al. Circulation 2009]. When the results were further analyzed, “important lessons emerged,” said Dr. Cohen. Patients who had markedly high aPTTs in response to heparin were more likely to be older, or female, have low body weight, and have renal insufficiency. These variables should be familiar because they are the same as those that are associated with an increased risk for bleeding.
When low–molecular–weight heparin was evaluated as an alternative to UFH, several advantages were identified. “Clinical data are available with enoxaparin in all types of ACS, with better outcomes than for UFH,” said Gilles Montalescot, MD, Pitié-Salpêtrière University Hospital, Paris, France. In a meta–analysis of six studies that involved patients with NSTEMI or STEMI, enoxaparin led to significantly lower rates of death or reinfarction (p=0.043) with no excess of major bleeding (p=0.42) [Murphy S et al. Eur Heart J 2007].
For patients who were treated with fibrinolysis for STEMI, enoxaparin significantly reduced death or reinfarction at 30 days compared with UFH (9.9% vs 12.0%; RR, 0.83; 95% CI, 0.77 to 0.90; p<0.0001), whether patients underwent subsequent percutaneous coronary intervention (PCI) or not [Antman EM et al. N Engl J Med 2006]. There was a significantly higher rate of TIMI major bleeding (2.1% vs 1.4%; p<0.001) but not intracranial hemorrhage. The drug became the standard of care for patients who were receiving fibrinolysis.
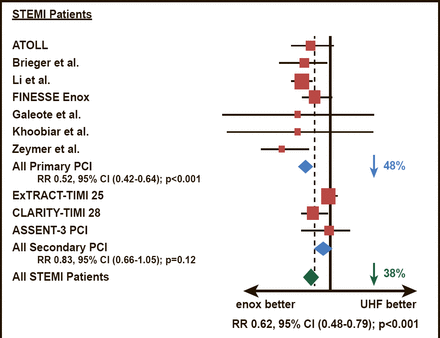
Enoxaparin and UFH were compared head–to–head among patients who were scheduled to have primary PCI in the ATOLL study [Montalescot G et al. Lancet 2011]. The results demonstrated that enoxaparin (intravenous 0.5 mg/kg bolus) was associated with a 17% reduction of the primary endpoint of death, complication of MI, procedure failure, or major bleeding (28% vs 33.7%; p=0.06). The main secondary endpoint (death, recurrent ACS, or urgent revascularization) was significantly lower among patients who were treated with enoxaparin compared with UFH (6.7% vs 11.3%; p=0.01). The study represented the first time that two–thirds of patients had primary PCI with radial access, which reduced major bleeding, blunting the reduction of the combined efficacy and safety primary endpoint with enoxaparin. In a subsequent meta–analysis, enoxaparin was superior to UFH in reducing mortality and bleeding outcomes during PCI, particularly among patients with STEMI (Figure 1) [Silvain J et al. BMJ 2012].
Mortality in Meta Analysis.
Reproduced from the British Medical Journal, Silvain J et al, 344:e553, 2012 with permission from BMJ Publishing Group Ltd.
The 2011 American College of Cardiology Foundation/American Heart Association (ACCF/AHA) guidelines for PCI indicate that enoxaparin and UFH should not be given together; the combination is harmful due to an excess of major bleeding [Levine GN et al. Circulation 2011].
Prof. Montalescot concluded, “Given its wide availability and low cost, enoxaparin is an attractive strategy to improve outcomes in the large number of patients undergoing PCI worldwide.”
Another anticoagulant, fondaparinux, may be used instead of enoxaparin. In the context of an early invasive or conservative strategy, fondaparinux is a Class I, level A recommendation in the European Cardiology Society guidelines for the management of NSTE–ACS [Hamm CW et al. Eur Heart J 2011]. In the 2011 focused update to the ACC/AHA guidelines for NSTE–ACS, fondaparinux is a Class I, level B recommendation in the setting of a conservative strategy [Wright RS et al. JACC 2011].
Bivalirudin represents a third and perhaps safer option for anticoagulation in ACS. This small–molecule agent has theoretical advantages over heparin in that it inhibits both fluid–phase and clot–bound thrombin and also inhibits rather than activates platelets as heparin does, explained Gregg W. Stone, MD, Columbia University Medical Center, Cardiovascular Research Foundation, New York, New York, USA.
Bivalirudin has been evaluated for use in both NSTE–ACS and STEMI, and studies have consistently shown lower rates of bleeding, with similar ischemic complications, yielding a favorable net clinical benefit outcome. The ACUITY study randomized 13,819 patients with moderate–high risk ACS who were undergoing an early invasive strategy to one of three groups: UFH or enoxaparin plus a glycoprotein IIb/IIIa (GP) inhibitor (the standard of care at the time of the study), bivalirudin plus a GP inhibitor, or bivalirudin alone [Stone GW et al. N Eng J Med 2006]. There was no difference in the composite ischemia endpoint (death, MI, or a recurrent ischemia that necessitated repeat revascularization) at 30 days between the three groups (7.4% [heparin plus a GP inhibitor] vs 7.9% [bivalirudin plus a GP inhibitor] vs 8.0% [bivalirudin alone]), but bivalirudin alone was associated with the lowest rate of bleeding (5.7% [heparin plus a GP inhibitor] vs 5.3% [bivalirudin plus a GP inhibitor] vs 3.1% [bivalirudin alone]). Adding a GP inhibitor to bivalirudin was not beneficial, in terms of reducing either ischemia or bleeding.
Similar results have been reported in other studies of patients with NSTE–ACS and STEMI comparing bivalirudin alone with the combination of heparin plus a GP inhibitor [Stone GW et al. N Engl J Med 2008; Kastrati A et al. N Engl J Med 2011]. In higher risk patients with STEMI, a lower rate of bleeding events was found with bivalirudin but at the expense of more acute stent thrombosis (1.3% bivalirudin vs 0.3% heparin plus GP inhibitor; p<0.001) [Stone GW et al. N Eng J Med. 2008]. Long–term follow–up for this cohort showed a lower rate of all–cause mortality at 3 years with bivalirudin [Stone GW et al. Lancet 2011]. The mechanism for this mortality reduction is not well understood.
In–hospital mortality was also reduced with bivalirudin compared with heparin plus a GP inhibitor (3.2% vs 4.0%; p=0.011) in an analysis of the data for nearly 60,000 patients who had STEMI and primary angioplasty in the PREMIERE perspective database [Pinto DS et al. Circ Cardiovasc Qual Outcomes 2012]. Bivalirudin also reduced bleeding (6.9% vs 10.5%; p<0.0001) and transfusion (5.9% vs 7.6%; p<0.0001), and these reductions led to decreased length of stay and lower costs.
As a result of these studies, bivalirudin is one of the Class I antithrombotic therapies that are recommended by numerous practice guidelines for treatment of patients with either NSTEMI or STEMI who are to have an early invasive strategy.
- © 2012 MD Conference Express®