Summary
DMTs for multiple sclerosis (MS) carry potentially serious risks, including opportunistic infections, altered response to vaccinations, development of cancer, and the appearance of autoimmune disorders. This article reviews safety data from clinical studies and post-marketing surveillance of DMTs for MS, as well as discusses the risks associated with such new therapies.
- Demyelinating Diseases
- Neurological Autoimmune Diseases
- Rheumatological Autoimmune Disorders
Adequately Assessing Risk Management of New Disease Modifying Therapies (DMT) is Difficult
DMTs for multiple sclerosis (MS) carry potentially serious risks, including opportunistic infections, altered response to vaccinations, development of cancer, and the appearance of autoimmune disorders. Jacek Losy, MD, PhD, Poznań University School of Medical Sciences, Poznań, Poland, reviewed safety data from clinical studies and post-marketing surveillance of DMTs for MS.
Phase 3 safety data from the AFFIRM [Polman CH et al. N Engl J Med 2006] and SENTINEL [Rudick RA et al. N Engl J Med 2006] studies of natalizumab show that the most common adverse events (AEs)—headache, fatigue, urinary tract infection, and arthralgia—were mild. Serious AEs were comparable to those observed with placebo. The rate of hypersensitivity reactions was 4% (0.8% serious reactions), and 6% of patients were persistently positive for antibodies to natalizumab. There was no increased risk of malignancies or depression. Two cases of progressive multifocal leukoencephalopathy (PML) and other opportunistic infections were reported. The risk of PML was 1/1000 over 18 months. Among all patients treated with natalizumab through January 2012 (n=96,582), 201 cases of PML have been reported. These data show that the key safety issues with natalizumab are hypersensitivity, immunogenicity, and PML and other opportunistic infections.
AEs associated with alemtuzumab include infusion reactions, infections, and malignancies. Autoimmune diseases have developed with long-term use in patients with diseases other than MS. Rituximab treatment in MS patients is associated with infusion reactions, infections, and grade IV ischemic coronary artery syndrome, malignant thyroid neoplasm, and symptoms of acute and progressive MS. Increased risk of PML and enteroviral infections is possible.
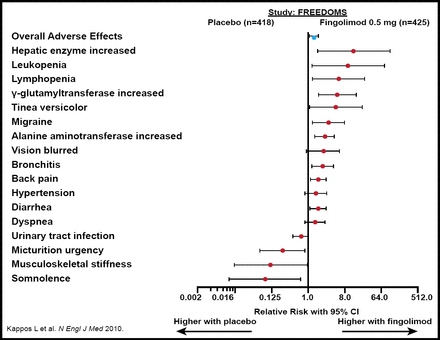
AEs reported with fingolimod are shown in Figure 1. ECG abnormalities were reported in more patients treated with fingolimod versus placebo or interferon beta-1a. Other potential complications of fingolimod therapy are latent DNA virus activation, bacterial infections, reversible posterior encephalopathy, and macular edema.
AEs: Fingolimod 0.5 mg Compared with Placebo.
Reproduced with permission from J. Losy, MD.
Prof. Losy concluded that clinical trials are of limited value for evaluating the safety of these drugs because of their limited duration and small numbers of strictly selected subjects. The full risk is better evaluated in post-marketing surveillance studies under actual clinical conditions.
Adequately Assessing Risk Management of New Disease-Modifying Therapies is Possible
A challenge with new DMTs for MS is determining the risk of AEs and weighing them against the benefits of treatment. According to Eva Havrdova, MD, Charles University, Prague, Czech Republic, risks of these new therapies can be assessed by discussing their risks and benefits, sharing information about prognostic factors, and creating risk management plans (RMPs) based on what has been learned about preventing AEs.
Currently, DMT is started early in patients with MS. However, the effectiveness of these drugs is only 30% to 60%; 62% to 75% of patients relapse and 20% to 27% worsen by ≥1 EDSS point within 2 years. Sormani et al. [Neurology 2011] showed that 2-year disability progression is entirely mediated by treatment-induced reduction in the number of MRI active lesions and relapses during the first year of therapy.
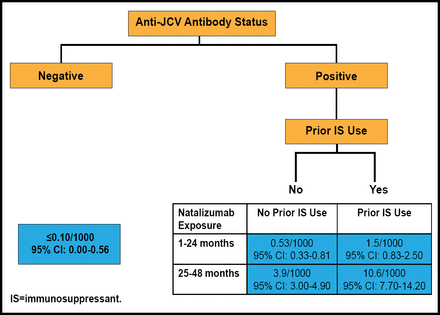
As of June 1, 2011, the Tysabri® (natalizumab) Observational Program [TOP Registry; NCT00477113] had amassed data on 3484 patients from 15 countries. Overall, the patients received a mean (SD) of 14.6 (11.21) and a median of 13.0 (range, 1 to 53) natalizumab infusions. A PML risk stratification tool developed through analysis of TOP registry data uses anti-JCV antibody status, prior immunosuppressant use, and treatment duration to determine the risk of developing PML (Figure 2). This tool shows that the risk for developing PML ranges from <0.10/1000 (95% CI, 0 to 0.56) in patients without anti-JCV antibodies to 10.6/1000 (95% CI, 7.7 to 14.2) in patients with anti-JCV antibodies, prior immunosuppressant use, and 25 to 48 months' exposure to natalizumab.
PML Risk Stratification Tool.
Reproduced with permission from E. Havrdova, MD.
A registry has not yet been established to track fingolimod safety issues, but the EMEA has recently issued additional security measures [http://www.ema.europa.eu/docs/en_GB/document_library/Medicine_QA/2012/04/WC500125689.pdf]. A change in RMP is expected from the European Medicines Agency. With respect to alemtuzumab, an RMP will be developed to avoid occurrences of idiopathic thrombocytopenic purpura. The RMP will include a recommendation on how to check thyroid function. PML has been shown to occur in 1/28,000 patients treated with rituximab.
Prof. Havrdova concluded that physicians should have a balanced discussion with their patients regarding the risks of untreated MS versus the risks of therapy. Well informed patients are better equipped to decide what risks they are willing to take. The risks of DMTs can be minimized by carefully following the RMP and monitoring patient adherence, treatment effects, and AEs.
The editors would like to thank the many members of the 6th World Congress on Controversies in Neurology presenting faculty who generously gave their time to ensure the accuracy and quality of the articles in this publication.
- © 2012 MD Conference Express®