Summary
Multiple sclerosis (MS) affects more than 350,000 people in the United States (US) and 2.5 million people worldwide. In the US alone, the health care costs that are associated with the treatment of MS are estimated to be more than $10 billion annually. Recently approved or investigational agents in advanced development have the potential to significantly improve outcomes for patients with relapsing-remitting MS [Fox EJ, Rhoades RW. Curr Opin Neurol 2012].
- Featured Meeting - Specialty page
- Demyelinating Diseases
Multiple sclerosis (MS) affects more than 350,000 people in the United States (US) and 2.5 million people worldwide. In the US alone, the health care costs that are associated with the treatment of MS are estimated to be more than $10 billion annually [www.clevelandclinicmeded.com]. Recently approved or investigational agents in advanced development have the potential to significantly improve outcomes for patients with relapsing-remitting MS (RRMS) [Fox EJ, Rhoades RW. Curr Opin Neurol 2012].
Alemtuzumab
Eva Havrdova, MD, PhD, Charles University, Prague, Czech Republic, discussed alemtuzumab, an anti-CD52 monoclonal antibody with remarkable efficacy in relapsing MS (RMS) [Perumal JS et al. Mult Scler 2012]. Alemtuzumab reduces circulating B and T cells that are suspected of playing a major role in the pathogenesis of MS.
In the CAMMS223 Phase 2 study [CAMMS223; NCT00050778], 12 mg of alemtuzumab was superior to subcutaneous interferon beta-1a (SC IFNB-1a) over 3 years [CAMMS223 Investigators. N Engl J Med 2008].
Outcomes included a 67% reduction in relapse rate (p<0.0001), with 76% of alemtuzumab patients relapse-free compared with 50% of those on SC IFNB-1a (p<0.0001); and 8% of alemtuzumab patients had sustained accumulation of disability (SAD) versus 27% of SC IFNB-1a patients (p=0.0006). Treatment effects were observed early in the trial and sustained through 5 years of follow-up [Coles AJ et al. ECTRIMS 2010], suggesting durability of effect. The safety profile was consistent with previous reports at up to 6.7 years (median 5 years) of follow-up.
Two Phase 3 trials of alemtuzumab, CARE-MS I [CARE-MS I; NCT00530348] and CARE-MS II [CARE-MS II; NCT00548405] recently were completed. Both were 2-year studies measuring relapse rate and disability progression.
CARE-MS I indicated a significant reduction in relapse rate over 2 years (p<0.0001) in the alemtuzumab group. No significant difference was observed between treatments (IV alemtuzumab vs SC IFNB-1a) for sustained accumulation of disability (SAD) or other Expanded Disability Status Scale (EDSS)-based endpoints [Coles AJ et al. ECTRIMS 2011].
Initial analysis of results in CARE-MS II showed a 49% reduction in relapse rate in patients receiving alemtuzumab 12 mg versus SC IFNB-1a (p<0.0001) and a 42% risk reduction in 6-month SAD (on EDSS; p=0.0084). Risks identified included infusion-associated reactions, infections, and antibody-mediated autoimmunity. Monitoring facilitated early detection and intervention.
Fingolimod
Fingolimod is a sphingosine-1 phosphate (S1P) receptor modulator and the first oral disease-modifying therapy to be approved in the European Union, the United States, and several other countries for the treatment of RRMS [Kipp M, Amor S. Mult Scler 2012; Ingwersen J et al. Clin Immunol 2012]. Hans-Peter Hartung, MD, Heinrich-Heine University, Düsseldorf, Germany, reported on the mechanisms of action and study results on fingolimod.
Acting as a functional antagonist at the S1P1 receptors, fingolimod selectively retains different subsets of T cells in lymphoid organs, preventing egress into the bloodstream. Thus, it limits the entry of pathogenic T cells into the central nervous system (CNS) parenchyma [Kipp M, Amor S. Mult Scler 2012].
Fingolimod crosses the blood-brain barrier, potentially acting at S1P receptors on neural cells in the CNS to mitigate neuropathologic processes associated with MS [Scott LJ. CNS Drugs 2011; Aktas O et al. Nat Rev Neurol 2010]. Recent studies suggest that it might play a role in remyelination and repair within the brain [Kipp M, Armor S. Mult Scler 2012].
In the 2-year, multinational FREEDOMS trial [Kappos L et al. N Engl J Med 2010], 24 months of treatment with fingolimod 0.5 or 1.25 mg/day was significantly better than placebo in terms of annualized relapse rate (ARR), the primary endpoint. Compared with placebo, the ARR was reduced by 55% in the fingolimod 0.5 mg/day group and by 60% in the fingolimod 1.25 mg/day group (p<0.0001 for both).
In the TRANSFORMS trial [Cohen JA. N Engl J Med 2010], the ARR (primary endpoint) was significantly lower with fingolimod 0.5 or 1.25 mg/day than with intramuscular IFNß-1a 30 μg once weekly, with a reduction in the ARR of approximately 40% to 50% in the fingolimod groups relative to the IFNß-1a group (p<0.001 for all).
Laquinimod
Ariel Miller, MD, PhD, Technion - Israel Institute of Technology, Haifa, Israel, presented data on laquinimod, an oral quinolone-3 carboxamide small molecule that suppresses inflammation, demyelination, and axonal damage in myelin oligodendrocyte-induced experimental autoimmune encephalomyelitis.
In vitro laquinimod down-regulates secretion of proinflammatory cytokines and enhances production of anti-inflammatory cytokines from peripheral blood mononuclear cells from healthy subjects and individuals with untreated RRMS. In addition, patients treated with laquinimod demonstrated up-regulation of serum brain-derived neurotrophic factor [Brück W, Wegner C. J Neurol Sci 2011].
Laquinimod has been evaluated in the Phase 3 ALLEGRO trial [Comi G et al. N Engl J Med 2012], a 2-year double-blind, placebo-controlled study that randomized 1106 patients with RRMS to receive either 0.6 mg laquinimod once daily or placebo. The primary outcome measure was the number of confirmed relapses.
Laquinimod treatment compared with placebo was associated with a modest reduction in the mean (±SD) annualized relapse rate (0.30±0.02 vs 0.39±0.03; p=0.002) and with a reduction in the risk of confirmed disability progression (11.1% vs 15.7%; HR, 0.64; 95% CI, 0.45 to 0.91; p=0.01). The mean cumulative numbers of gadolinium-enhancing (Gd+) lesions and new or enlarging lesions on T2-weighted images were lower for patients receiving laquinimod than for those receiving placebo (1.33±0.14 vs 2.12±0.22 and 5.03±0.08 vs 7.14±0.07, respectively; p<0.001 for both comparisons). Transient elevations in alanine aminotransferase levels to greater than three times the upper limit of the normal range were observed in 24 patients receiving laquinimod (5%) and eight receiving placebo (2%) [Comi G et al. N Engl J Med 2012].
In a second Phase 3 study, BRAVO [BRAVO; NCT00605215], 0.6 mg laquinimod was compared with placebo and IFNB1-a (intramuscular injection) in approximately 1200 RRMS patients.
Killestein et al. [Lancet Neurol 2011] noted that the primary endpoint—decrease in annual relapse rate for laquinimod versus placebo—was not significant. However, there were significant reductions in extended disability status scale progression (33.5%; p=0.044) and loss of brain volume (27.5%; p<0.0001).
According to Prof. Miller, laquinimod has modest effects on disease activity (relapses and MRI lesion counts), robust effects on disease burden (progression of disability and MRI-measured irreversible tissue damage), and significant reductions of fatigue and amelioration of quality of life limitations.
Ocrelizumab
David Leppert, MD, University Hospital, F. Hoffman,-La Roche Ltd., Basel, Switzerland, presented data on ocrelizumab, a recombinant humanized antibody designed to selectively target CD20 B cells [Kappos L et al. Lancet 2011].
The recent successful targeting of B cells in patients with MS using monoclonal antibodies targeting CD20 has established that it is no longer a question of if B cells contribute, but how they contribute to MS disease activity [Barun B, Bar-Or A. Clin Immunol 2011].
Ocrelizumab binds to a different but overlapping epitope compared with rituximab and appears to deplete B cells primarily through antibody-dependent cellular toxicity rather than by a complement-dependent mechanism. This might offer an improved efficacy profile with fewer infusion-related reactions [Kausar F et al. Expert Opin Biol Ther 2009].
The recently completed 24-week, placebo-controlled and active comparator, multicenter Phase 2 study of ocrelizumab in RRMS, randomized 220 patients to one of four arms: ocrelizumab 600 mg or 2000 mg over two infusions (at Days 1 and 15); placebo; or interferon beta-1a, 30 μg intramuscular injection weekly (as an open-label arm) [Barun B, Bar-Or A. Clin Immunol 2011]. The primary objective was to investigate the effect of ocrelizumab on the total number of Gd+ T1 lesions observed on brain MRI scans for Weeks 12, 16, 20, and 24 versus placebo [Kappos L et al. Lancet 2011].
Results demonstrated highly significant differences in the primary endpoint of total number of Gd+ T1 lesions for both ocrelizumab doses versus placebo (p<0.0001 for both), with relative reductions of 89% for the 600-mg arm and 96% for the 2000-mg arm. The majority of ocrelizumab-treated patients had no clinical disease activity through Week 96, and the safety profile was acceptable, with no reports of opportunistic infections detected up to 96 weeks.
Ocrelizumab is currently in Phase 3 development for the treatment of patients with RMS and primary progressive MS (PPMS).
Teriflunomide
Teriflunomide is an oral disease-modifying therapy for relapsing forms of MS. Mark Freedman, MD, Ottawa, Ontario, Canada, discussed its mechanism of action and results from the Phase 3 Teriflunomide Multiple Sclerosis Oral Trial (TEMSO) [O'Connor P et al. N Engl J Med 2011].
According to Dr. Freedman, teriflunomide blocks de novo pyrimidine synthesis, reducing T- and B-cell proliferation in response to auto-antigens. Cells that live on the existing pyrimidine pool (eg, hematopoietic and memory T cells) are unaffected. The agent has minimal CYP450 involvement.
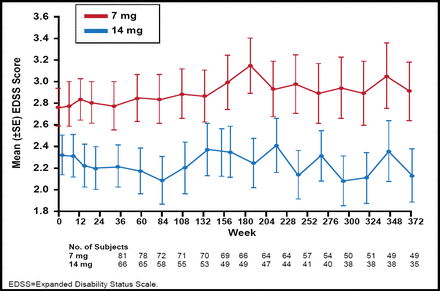
The Phase 2 teriflunomide study showed that unique active lesions were reduced by 61.1% with teriflunomide 7 mg/day (p<0.03) and by 61.3% by teriflunomide 14 mg/day (p<0.01) versus placebo [O'Connor P et al. Neurology 2006]. Teriflunomide was well tolerated and demonstrated a favorable safety profile. A long-term open label extension study showed that after 9 years of teriflunomide treatment, there were only minimal changes in disability progression, as shown by the mean change from baseline expanded disability status scale (EDSS; Figure 1) [Confavreux C et al. Mult Scler 2012]. Lower EDSS scores were observed in the teriflunomide 14 mg versus 7 mg group.
Mean EDSS Score (for the pooled core study and the extension).
Reproduced with permission from Sage Publications Ltd. Confavreux C et al. Long-term follow-up of a phase 2 study of oral teriflunomide in relapsing multiple sclerosis: safety and efficacy results up to 8.5 years. Multiple Sclerosis 2012.
The Phase 3 teriflunomide program was initiated following successful completion of the Phase 2 study and is one of the largest worldwide, involving 1088 people with relapsing forms of MS from 126 centers in 26 countries. Key inclusion criteria included EDSS score <5.5 and ≥1 relapse in the previous year or ≥2 relapses in the previous 2 years. Patients were randomized to placebo (n=363), teriflunomide 7 mg (n=365), or teriflunomide 14 mg (n=358) for 108 weeks. After this phase, placebo patients were re-allocated to teriflunomide 7 mg or 14 mg. The primary endpoint was annualized relapse rate. The secondary endpoints were time to sustained disability progression and MRI burden of disease.
The TEMSO Phase 3 results confirmed the Phase 2 findings. Compared with placebo, teriflunomide once daily significantly reduced annualized relapse rate (0.54 for placebo vs 0.37 for teriflunomide at either 7 mg or 14 mg), with relative risk reductions of 31.2% and 31.5%, respectively (p<0.001 for both comparisons with placebo) [O'Connor P et al. N Engl J Med 2011]. The risk of confirmed disability progression at 12 weeks was significantly reduced with teriflunomide 14 mg versus placebo (hazard ratio reduction 29.8%; p=0.0279). Subjects treated with teriflunomide 7 mg had a hazard ratio reduction of 23.7% (p=0.0835) versus placebo.
Key MRI data showed that teriflunomide 14 mg reduced the relative increase in burden of disease by 67.4% versus placebo (p=0.0003). Compared with placebo, unique active lesions were reduced by 69.4% with teriflunomide 14 mg (p<0.0001) and by 47.7% with teriflunomide 7 mg (p<0.0001). Both teriflunomide doses reduced white matter volume loss (164.3% reduction 14 mg vs placebo; p=0.0002).
Teriflunomide was generally well-tolerated. Adverse events occurred at a higher rate in the teriflunomide versus placebo groups and included diarrhea, nausea, and alanine transferase increases. No serious infections occurred in patients treated with teriflunomide [Fox EJ, Rhoades RW. Curr Opin Neurol 2012].
- © 2012 MD Conference Express®