Summary
Small subcortical strokes, also known as lacunar strokes, constitute more than 25% of brain infarcts and are the most common cause of vascular cognitive impairment. How to optimally prevent stroke recurrence and cognitive decline in patients with small subcortical stroke is unclear [Benevente OR et al. Int J Stroke 2011]. This article discusses results from The Secondary Prevention of Small Subcortical Strokes Study: The Antiplatelet Trial Results [SPS3; NCT00059306].
- Cerebrovascular Disease Clinical Trials
- Prevention & Screening
- Episodic & Paroxysmal Disorders
Small subcortical strokes, also known as lacunar strokes, constitute more than 25% of brain infarcts and are the most common cause of vascular cognitive impairment. How to optimally prevent stroke recurrence and cognitive decline in patients with small subcortical stroke is unclear [Benevente OR et al. Int J Stroke 2011]. Oscar R. Benavente, MD, FRCP(C), University of British Columbia, Vancouver, British Columbia, Canada, presented results from The Secondary Prevention of Small Subcortical Strokes Study: The Antiplatelet Trial Results [SPS3; NCT00059306].
SPS3 was a randomized, double-blind, multicenter, investigator-initiated, international trial that was conducted at 81 clinical sites in eight countries. From March 2003 to April 2011, 3020 patients with symptomatic lacunar strokes in the prior 6 months verified by magnetic resonance imaging were randomized in a 2 × 2 factorial design to antiplatelet therapy—325 mg aspirin daily plus 75 mg clopidogrel daily versus 325 mg aspirin daily plus placebo—and to one of two levels of open-label blood pressure targets—intensive (130 mm Hg) versus usual (130 to 149 mm Hg). Exclusion criteria included cortical stroke, cardioembolic disease, or carotid stenosis.
SPS3 was a superiority trial that was powered to detect a clinically significant difference in outcomes. The primary outcome was time to recurrent stroke (ischemic or hemorrhagic), analyzed separately for each intervention. The secondary outcomes were rates of cognitive decline and major vascular events. Mean time from index event to randomization was 76 days. Mean follow-up was 3.5 years. Loss to follow-up was 2%. The primary and secondary outcomes were centrally, blindly adjudicated.
Baseline characteristics of patients were similar in both groups, with a mean Mini Mental State Exam score of 28± 2.3. In the aspirin group (n=1503), 66% had a Rankin score of 0 to 1; the figure in the aspirin+clopidogrel group (n=1517) was 67%. The race/ethnicity of participants was 52% white, 31% Hispanic, and 17% black. Baseline medical characteristics, clinical syndromes, and percentage from each region (North America, Latin America, and Spain) were all similar.
The probability of the primary event over time showed no difference between the two groups: aspirin=138 (2.7%/patient-year) compared with aspirin+clopidogrel (n=126; 2.5%/patient-year; HR, 0.92; 0.73 to 1.2; p=0.52). The incidence of ischemic stroke was also nonsignificant: aspirin group (n=125; 2.4%/patient-year) compared with aspirin+clopidogrel (n=105; 2.1%/patient-year; HR, 0.85; 0.66 to 1.1; p=0.21). Major vascular events (stroke, myocardial infarction, or vascular death) were also not significantly different.
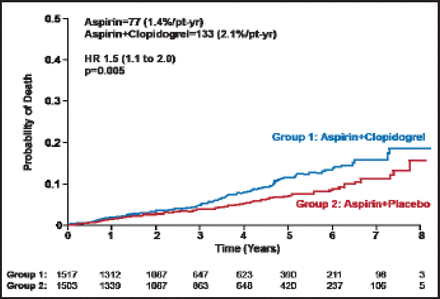
Differences in all-cause mortality were significantly different: aspirin (n=77; 1.4%/patient-year) and aspirin+clopidogrel (n=113; 2.1%/patient-year; HR, 1.5; 1.1 to 2.0p=0.005; Figure 1). Differences in probable vascular events (p=0.012), all hemorrhages (p<0.001), and non-CNS hemorrhages were also significant (p<0.001; Table 1).
Major Hemorrhages.
All-Cause Mortality.
Reproduced with permission from OR Benavente, MD, FRCP(C).
The antiplatelet intervention was stopped prematurely in July 2011 for reasons of safety and futility. The authors concluded that dual antiplatelet therapy was not more efficacious than aspirin alone. Major bleeds and total mortality were increased. The results do not support the use of combination therapy for stroke prevention in patients with lacunar strokes.
- © 2012 MD Conference Express®