Summary
Prior studies indicate that genetic factors are important in the formation and rupture of intracranial aneurysms [Broderick JP et al. BMC Med Genet 2005]. This article discusses the initial results from the Familial Intracranial Aneurysm Study II [FIA II; NCT00071565].
- Ischemia Clinical Trials Genomics
Prior studies indicate that genetic factors are important in the formation and rupture of intracranial aneurysms (IAs) [Broderick JP et al. BMC Med Genet 2005]. Joseph P. Broderick, MD, University of Cincinnati, Cincinnati, Ohio, USA, presented initial results from the Familial Intracranial Aneurysm Study II [FIA II; NCT00071565].
Most deaths from subarachnoid hemorrhage (SAH) are due to rapid and massive brain injury from the initial bleeding; therefore, prevention of aneurysm formation is of paramount importance. Scientific evidence suggests that a genetic component plays an important role in the development of IAs, but the specific genes that are involved have not been identified.
The purposes of FIA II were to identify genes that may increase the risk of aneurysm development in the brain and to determine the effect of environmental factors, such as cigarette smoking and high blood pressure, on the expression of those genes.
According to Dr. Broderick, approaches to dissect the genetic contribution to IAs have had mixed results. Linkage has been largely unsuccessful and, to this point, has not identified genetic variants that are associated with a risk of IA. Genomewide association studies (GWAS) have been somewhat successful, identifying several replicated genes with modest effect size (OR approximately 1.2 to 1.3), but again have yet to identify specific variants within these genes that are causally related to IA.
Whole exome sequencing (WES) is a promising new approach that focuses on the coding region of the genome (the exome). This method is designed to find variants that affect protein structure or function, which are relatively common in Mendelian disorders. WES complements GWAS but is best suited to the identification of rare (r) variants of moderate to large effect size, while GWAS finds common variants that are typically of smaller effect size.
The authors reviewed families with a dense history of IA and available DNA. Seven families that met their criteria (two or more affected pairs of siblings, three or more family members with IA, or individuals with a confirmed IA but without a family history) were identified. DNA from affected individuals was sent to the Center for Inherited Disease Research (CIDR) (ie, 32 exomes from 32 individuals who were sequenced).
Quality filtering produced 93,635 single-nucleotide variants (SNVs). Biologic filters, which retain only the variants that meet the biological hypothesis (ie, rare exonic and amino acid-altering alleles that segregate in Mendelian fashion may contribute to IA pathology), reduced that figure to 871 SNVs. Variants were kept if they were observed in at least three affected individuals in a single family if the SNVs were autosomal dominant or recessive. Of the 871 SNVs, 31 were in the gene otology pathways of interest, such as collagen.
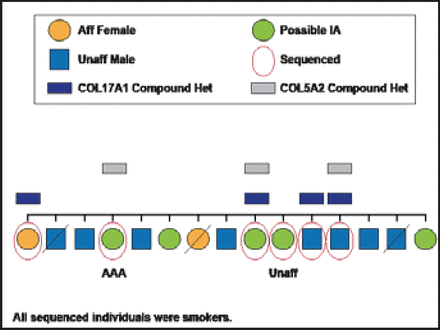
Variants in two collagen genes were identified in at least three family members in one of these IA families (Figure 1). These variants are predicted to potentially cause abnormal function in these proteins. Collaborators from Poland have demonstrated that both of these genes are expressed differently in aneurysmal tissue as compared with vascular tissue of the middle meningeal arteries. Figure 2 shows the presence of these variants in affected family members, all of whom were also smokers—the most important environmental risk factor for IA. Further work will need to be done to see whether these gene variants are truly causal in the development of IA. Ultimately, the interplay of three factors—environment (smoking, hypertension), common variants of individual small effects (GWAS), and rare variants with medium to large effects (WES)—likely contributes to disease susceptibility.
Collagen Variants.
Reproduced with permission from J. Broderick, MD.
One Family and Collagen.
Reproduced with permission from J. Broderick, MD.
The identification of susceptibility genes, along with a better understanding of environmental interactions, such as cigarette smoking, may prevent the development of IAs and IA ruptures in people who are at risk for this condition.
- © 2012 MD Conference Express®