Summary
As landmark trials have shown no outcome improvement with vascular bypass or percutaneous angioplasty and stenting, aggressive medical management remains the gold standard for symptomatic intracranial artery disease [Augoustides JG. J Cardiothorac Vasc Anesth 2012]. This article presented outcomes of patients in the SAMMPRIS trial who had failed antithrombotic therapy at study enrollment.
- Cerebrovascular Disease Clinical Trials
- Thrombotic Disorders
- Interventional Techniques & Devices
As landmark trials have shown no outcome improvement with vascular bypass or percutaneous angioplasty and stenting, aggressive medical management (AMM) remains the gold standard for symptomatic intracranial artery disease [Augoustides JG. J Cardiothorac Vasc Anesth 2012]. Helmi L. Lutsep, MD, FAHA, Oregon Health & Science University, Portland, Oregon, USA, presented outcomes of patients in the SAMMPRIS trial who had failed antithrombotic therapy at study enrollment.
The analysis compared AMM and percutaneous transluminal angioplasty and stenting (PTAS) in group-on and group-off antithrombotic therapy at the qualifying event. The primary endpoints were stroke and death at 30 days or stroke in the territory of the qualifying artery beyond 30 days.
Data showed that 63% of SAMMPRIS patients (284/451) had their qualifying events while on antithrombotic therapy; 140 were randomized to AMM, and 144 were randomized to PTAS. Thirty-seven percent of patients (167/451) were not on antithrombotic therapy; 87 were randomized to AMM, and 80 were randomized to PTAS. Of the 284 patients who had a qualifying event on antithrombotics, 95.8% were on antiplatelet therapy only (clopidogrel+aspirin; 22.5%), 1.4% were taking anticoagulants only, and 2.8% were on both antiplatelet and anticoagulant therapy.
The patient characteristics were different in the group with a qualifying event on antithrombotic therapy compared with the group that was off of antithrombotic therapy. The group that had been on antithrombotic therapy at the time of the qualifying event was older and had a longer history of hypertension, lipid disorders, coronary artery disease, and stroke prior to the qualifying event. In this group, the qualifying event was also more often a stroke rather than a transient ischemic attack, and the symptomatic artery differed, more commonly involving a vertebral or basilar artery.
Baseline measures of risk factors between the two groups were similar, except that glycated hemoglobin in diabetics was lower in the antithrombotic therapy group (p=0.03). In terms of concomitant medication at the time of study entry, only beta-blockers and nonstatin lipid-lowering medications were different between the group-on (119/284, 42%; 58/284, 20%) and group-off (53/167, 32%; 19/167, 11%) participants (p=0.0319 and p=0.0137, respectively).
The percentage of group-on patients who were on AMM (n=140) who reached the primary endpoint was 12.1%; in the PTAS group (n=144), the primary endpoint was reached by 21.5% of patients. At 30 days, the primary endpoint rates were 4.3% versus 16.0%, respectively; after 30 days, they were 7.9% versus 5.6%, respectively.
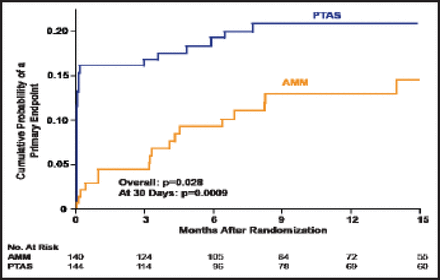
Among those who were on antithrombotic therapy with a qualifying event, the cumulative probability of a primary endpoint occurring at 15 months of follow-up was significantly higher in the PTAS group than the AMM group (p=0.028 overall; p=0.0009 at 30 days; Figure 1). Among those who were on antithrombotic therapy and had a history of ischemic stroke with a qualifying event, the difference in the primary endpoint between the AMM (n=49) and PTAS group (n=51) was 14.3% versus 35.3%, respectively. At 30 days, the respective numbers were 8.2% versus 25.5%, and after 30 days, they were 6.1% versus 9.8% (p=0.014 overall; p=0.019 at 30 days).
Qualifying Event On Antithrombotic Therapy.
Reproduced with permission from HL Lutsep.
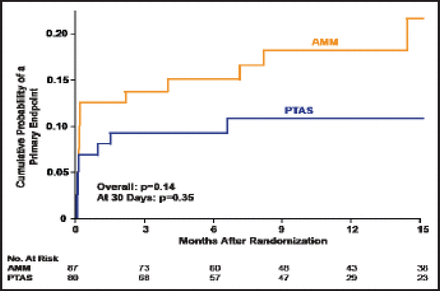
There was no significant difference between the AMM and PTAS groups that had a qualifying event while not on antithrombotic therapy (p=0.14 overall; p=0.35 at 30 days; Figure 2). No significant difference was observed between those patients who were treated with AMM who were either on or off antithrombotics at the time of their qualifying event.
Qualifying Event Not On Antithrombotic Therapy.
Reproduced with permission from HL Lutsep.
The authors pointed out that those patients who had been on antithrombotics had a greater number of risk factors and that those patients with intracranial stenosis had more benefit from AMM than PTAS with the Wingspan stent system, even if they had failed antithrombotic therapy.
The benefit of AMM is similar in patients who are on versus off antithrombotic medication at the time of their qualifying events. These findings support those from the Warfarin Aspirin Symptomatic Intracranial Disease (WASID) trial, showing that antithrombotic therapy failure does not identify a higher-risk subgroup of patients with intracranial stenosis [Chimowitz MI et al. N Engl J Med 2005].
- © 2012 MD Conference Express®