Summary
The central nervous system plays a key role in the regulation of energy balance, particularly through the activities of a group of neurons—neuropeptide Y [NPY]/agouti-related protein [AgRP] and pro-opiomelanocortin [POMC] in the arcuate nucleus of the hypothalamus and are sensitive to information that comes from hormones (eg, ghrelin, insulin, leptin) and nutrients, such as glucose. This article discusses whether this region is also capable of sensing fatty acids.
- Diabetes Mellitus
- Obesity
The central nervous system (CNS) plays a key role in the regulation of energy balance, particularly through the activities of a group of neurons—neuropeptide Y [NPY]/agouti-related protein [AgRP] and pro-opiomelanocortin [POMC] in the arcuate (ARC) nucleus of the hypothalamus and are sensitive to information that comes from hormones (eg, ghrelin, insulin, leptin) and nutrients, such as glucose. In his presentation, Christophe Magnan, PhD, University Diderot, Paris, France, discussed whether this region is also capable of sensing fatty acids.
The ability of the brain to sense fatty acids was first suggested in 1975 by Oomura et al., when they noted that the release of free fatty acids into the bloodstream of energy-deficient rats caused feeding activity [Physiol Behav 1976]. Evidence has continued to mount, including a study by Wang et al. that demonstrated an interaction between glucose and fatty acids that regulated oleic acid sensing in ARC neurons [J Neurophysiol 2006] and another by Migrenne et al., who noted that the presence of oleic acid ‘inhibited’ neurons in the ARC nucleus, the effect of which they were able to reverse using tolbutamide [Diabetes 2006]. In another study, administration of a PPARα agonist into the hypothalamus of fatty acid synthase (FAS) knockout mice increased PPARα target genes and normalized food intake, suggesting a critical role for brain FAS in the regulation of feeding. Increased physical activity was also noted and appeared to be mediated through the provision of ligands that were generated by FAS to PPARα [Chakravarthy MV et al. J Clin Invest 2007].
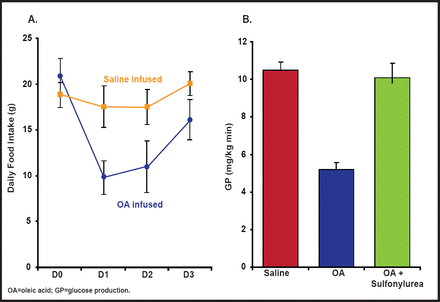
Central administration of oleic acid has been shown to markedly inhibit hepatic glucose production and food intake, while administration of inhibitors of ATP-sensitive K+ channels blunts the effect of oleic acid on hepatic glucose production. This suggests that fatty acids can signal nutrient availability to the CNS—for example, during a meal—which in turn limits further delivery of nutrients to the circulatory system (Figure 1) [Obici S et al. Diabetes 2002]. FAT/CD36, a fatty acid transporter that can alter cell function independently of intracellular fatty acid metabolism, may also be involved in fatty acid signaling, as evidenced by Le Foll et al. [Am J Physiol 2010]. Ghrelin, which normally increases food intake, also seems to require the presence of fatty acids to relay its effect, as evidenced by a report from Lopez et al. that shows that the effect of ghrelin on food intake is decreased when fatty acid metabolism is impaired by the presence of the CPT1 inhibitor etomoxir [Cell Metab 2008].
Central Administration of Oleic Acid Inhibits Glucose Production and Food Intake.
Reproduced with permission from the American Diabetes Association. Central Administration of Oleic Acid Inhibits Glucose Production and Food Intake. Obicci S et al. Diabetes 2002; 51:271–275.
However, these metabolic issues become more complicated in the context of the absence or excess of adipose tissue. Adipose tissue sequesters dietary fat and thus protects other tissues from excess fat exposure, especially after meals. In a study in men, adipose tissue fatty acid handling was studied in response to three meals. The efficiency of adipose tissue fatty acid uptake increased robustly with each meal. Thus, human adipose tissue has a significant potential to upregulate fat storage during a normal day. This may provide an explanation for the beneficial properties of normal amounts of adipose tissue [Ruge T et al. J Clin Endocrinol 2009].
The inhibitory effect of fatty acids on food intake in response to a meal is probably not a direct consequence of plasma, since their concentration decreases during a meal. In contrast, increased plasma triglycerides (TGs) or TG-rich lipoproteins (such as very-low-density lipoprotein cholesterol) could be detected and locally hydrolyzed in the hypothalamus, thus providing fatty acids to sensitive neurons. Lipoprotein lipase (LPL), a serine hydrolase that releases free fatty acids from circulating TG-rich lipoproteins in neurons, has been shown to contribute to free fatty acid-mediated signaling in the brain. Decreased LPL activity in the ARC nucleus increases food intake in rats. Neuron-specific LPL-deficient mice are hyperphagic and become obese by 16 weeks of age [Wang H et al. Cell Metab 2011]. This effect is accompanied by elevations in the hypothalamic orexigenic neuropeptides AgRP and NPY and reductions in metabolic rate. The uptake of TG-rich lipoprotein is reduced in the hypothalamus of 3-month-old mice, with deficiencies in essential fatty acids in the hypothalamus evident by 3 months and major deficiencies of long-chain n-3 fatty acids by 12 months. These results indicate that TG-rich lipoproteins are sensed in the brain by an LPL-dependent mechanism and provide lipid signals for the central regulation of body weight and energy balance [Wang H et al. Cell Metab 2011].
An important question that concerns fatty acid sensing is whether lipid overload into the CNS induces a deregulation of insulin sensitivity. Marsollier et al. demonstrated that cerebral fatty acid overload induces an enhancement of neuronal hypothalamic nitric oxide synthase (nNOS) activity, which, at least in part, is responsible for deregulation of hypothalamic fatty acid sensing and consequently induces hepatic insulin resistance, which may partly contribute to impairment of glucose homeostasis [PLoS One 2009].
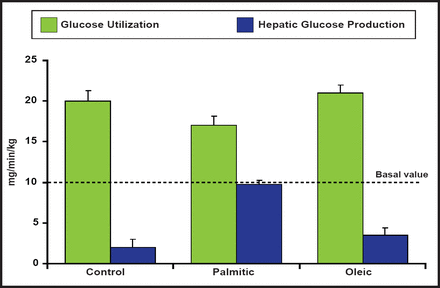
Insulin signaling can also be modulated by several isoforms of protein kinase C (PKC) in peripheral tissues. One specific form, PKC-θ, is expressed in discrete neuronal populations of the ARC nucleus, specifically the NPY/AgRP neurons and the dorsal medial nucleus in the hypothalamus that are critical for regulating the energy balance. Hypothalamic insulin resistance that is induced by palmitate (diets that are high in fat) leads to hepatic insulin resistance and increased localization of PKC-θ in the hypothalamus. These results suggest that many of the deleterious effects of high-fat diets, specifically those that are enriched with palmitic acid, are CNS-mediated via PKC-θ activation, resulting in reduced insulin activity (Figure 2) [Benoit SC et al. J Clin Invest 2009].
Reduced Insulin Activity Associated with High-Fat Diets.
Reproduced with permission from the American Society for Clinical Investigation. Palmitic acid mediates hypothalamic insulin resistance by altering PKC-θ subcellular localization in rodents. Benoit SC et al. J Clin Invest 2009; 119(9): 2577–2589.
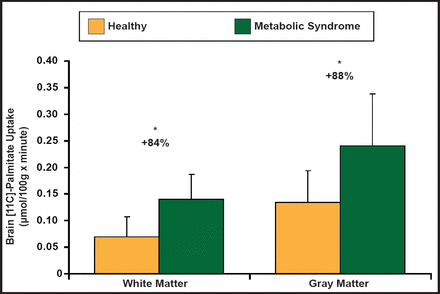
Although there are little data in humans for brain fatty acid sensing, a recent study has shown that brain fatty acid uptake is enhanced in obese individuals with metabolic syndrome (METS) compared with control subjects. Brain global fatty acid uptake, measured during fasting conditions using positron emission tomography (PET) with [(11)C]-palmitate and [(18)F] fluoro-6-thia-heptadecanoic acid ([(18)F]-FTHA), is 50% higher in patients with METS compared with control subjects. In the METS group, the nonoxidized fraction, measured using [(11)C]-palmitate, was 86% higher. Brain fatty acid uptake, measured with [(18)F]-FTHA-PET, was associated with age, fasting serum insulin, and homeostasis model assessment (HOMA) index. Both total and nonoxidized fractions of fatty acid uptake were associated with body mass index. Rapid weight reduction decreased brain fatty acid uptake by 17% (Figure 3) [Karmi A et al. Diabetes 2010].
Brain Fatty Acid Uptake.
Reproduced with permission from the American Diabetes Association. Increased Brain Fatty Acid Uptake in Metabolic Syndrome. Karmi A et al. Diabetes 2010; 59:2171–2177.
The evidence to date indicates that fatty acid-sensitive neurons that can excite or inhibit are present in the hypothalamus, at least in rodents. It is apparent that fatty acid sensing may participate in the regulation of energy balance in physiological conditions, while local lipolysis may also be a regulator of fatty acid sensing. Deregulation of brain lipid sensing may contribute to changes in nervous control of the body's energy balance. However, fatty acids are not the only agents that are involved in the nervous control of energy balance that affects our susceptibility to obesity and diabetes.
- © 2011 MD Conference Express®