Summary
This article addresses the relationship between the heart and kidney. Individuals with chronic kidney disease (CKD) are twice as likely to have cardiovascular disease (CVD) than individuals with normal kidney function, with 40% also being diagnosed with coronary artery disease and/or congestive heart failure. CVD progresses at twice the rate in those with CKD as in those without CKD.
- Cardiology Genomics
- Hypertension & Kidney Disease
- Prevention & Screening
- Renal Disease
- Myocardial Infarction
- Heart Failure
Between 8% and 10% of individuals within the Medicare and the dual-eligible Medicare/Medicaid programs have chronic kidney disease (CKD) or end-stage renal disease (ESRD) [Collins AJ et al. Am J Kidney Dis 2009]. CKD is defined by either a glomerular filtration rate (GFR) of less than 60 ml/min/1.73 m2 of body surface area or the presence of kidney damage, regardless of the cause, for 3 or more months. Together with ESRD, the two conditions account for more than one-third of overall expenditures in the two health care programs [Foundation NK. Am J Kidney Dis 2002].
Dick De Zeeuw, MD, PhD, University Medical Center, Groningen, The Netherlands, addressed the relationship between the heart and kidney. Individuals with CKD are twice as likely to have cardiovascular disease (CVD) than individuals with normal kidney function, with 40% also being diagnosed with coronary artery disease and/or congestive heart failure (CHF). CVD progresses at twice the rate in those with CKD as in those without CKD. In addition, patients with CKD are hospitalized for heart failure 5 times more often than those without CKD, while the major cause of death in patients with CKD is cardiovascular in nature [Collins AJ et al. Kidney Int Suppl 2003; Sarnak MJ et al. Am J Kidney Dis 2000].
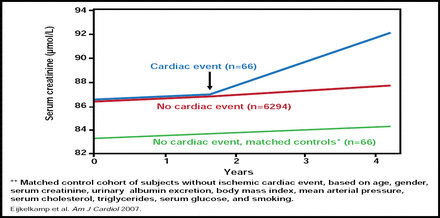
There is ample evidence that renal dysfunction predicts CVD progression. However, the impact of CVD on the progression of renal disease has not been studied extensively, and limited data are available from subgroup and/or post hoc analyses. In a secondary analysis from the Prevention of Renal and Vascular End-stage Disease (PREVEND) cohort, renal function declined more rapidly in patients after myocardial infarction (MI; p=0.005; Figure 1) [Eijkelkamp WB et al. Am J Cardiol 2007].
PREVEND: Effect of MI on Renal Function in General Population.
Reproduced with permission from D. De Zeeuw, MD, PhD.
In a post hoc analysis of the data from the Captopril and Thrombolysis Study (CATS), a significant decline in the estimated glomerular filtration rate (eGFR) was observed, beginning 3 days after an MI in patients with and without CHF (5.5 ml/min/yr vs 12 ml/min/yr, respectively) [Hillege HI et al. Eur Heart J 2003].
Renal Biomarkers for CV
There are two types of renal biomarkers that are associated with CV dysfunction. The first type includes markers, such as creatinine and cystatin C, that allow estimation of the filtration capacity of the kidney (GFR). Measurement of urine albumin represents a different type of renal biomarker, as it identifies impaired handling of proteins by the kidney that can be detected at an early stage of kidney dysfunction, particularly when microalbuminuria is assessed using sensitive assays.
Numerous studies have reported the value of serum creatinine or eGFR (estimated from serum creatinine or serum cystatin-C levels) as markers for CVD risk or all-cause mortality. In a seminal paper, Go et al. showed that an above mean GFR of 3 years in duration was strongly associated with all-cause mortality, with the risk of death increasing as GFR decreased below 60 ml/min/1.73 m2. The adjusted hazard ratio for death was 5.9, with an estimated GFR of less than 15 ml/min/1.73 m2 (95% CI, 5.4 to 6.5). A similar increase in the risk for CV events and hospitalization was also associated with declining GFR [Go AS et al. N Engl J Med 2004].
Gibson et al. analyzed data from the Thrombolysis In Myocardial Infarction (TIMI)-10, TIMI-14, and Intravenous nPA for the Treatment of Infarcting Myocardium Early (InTIME-II) trials, finding that 30-day mortality increased within each TIMI risk score for ST-elevation myocardial infarction (STEMI) as renal function declined. The odds ratio for 30-day mortality in patients with severe impairment (defined as a creatinine clearance <30 ml/min) was 3.73 (95% CI, 2.55 to 5.45; p<0.001) [Gibson CM et al. J Am Coll Cardiol 2003].
Cooper et al. found that renal function was associated with the risk of postoperative complications after coronary artery bypass surgery, with the mortality rate increasing from 1.3% for those with normal renal function to 9.3% for those with severe renal dysfunction who were not on dialysis and 9.0% for those who were on dialysis. The most powerful predictor of postoperative mortality and morbidity was preoperative GFR [Cooper WA et al. Circulation 2006].
An analysis of the Valsartan in Acute Myocardial Infarction Trial (VALIANT) found that the risk of death or the composite endpoint (death from CV causes, reinfarction, CHF, stroke, or resuscitation after cardiac arrest) increased with declining eGFR. Each 10-unit reduction in patients with GFR <81.0 ml/min/1.73 m2 was associated with a hazard ratio for death and nonfatal CV outcomes of 1.10 (95% CI, 1.08 to 1.12), regardless of treatment [Anavekar NS et al. N Eng J Med 2004].
Multiple studies have shown that albuminuria is related to CV outcome. This is true in the general population but also in several different disease populations, including hypertension, diabetes, and heart failure [Wachtell et al. Annals Int Med 2003; Yuyun et al. Diabetes Med 2003; Gerstein et al. JAMA 2001; de Zeeuw D et al. Circulation 2004].
Treatment?
More evidence for the relationship between kidney function and CVD comes from examining the benefits of treating parameters that are related to kidney function or to cardiac function and the impact of long-term renal and CV outcomes.
It is hard to treat GFR—the only way to do so is to transplant extra kidney tissue and see what the impact on CV morbidity and mortality is. Thus, the goal of therapy is to reduce further progression of disease and loss of kidney function with improvements in surrogate markers that are associated with outcomes, such as urine albumin.
The PREVEND Intervention (PREVEND-IT) trial found that treatment with the angiotensin-converting enzyme (ACE) inhibitor fosinopril significantly reduced mean urinary albumin excretion by 26% (p<0.001), simultaneously providing a 40% RRR in CV mortality and morbidity. Although the latter finding was not statistically significant, the study suggested that the mere treatment of patients (lowering their albuminuria) who have only slightly increased albuminuria is associated with better CV outcome (HR, 0.60; 95% CI, 0.33 to 1.10; p=0.098, log-rank) [Asselbergs FW et al. Circulation 2004]. Other trials found cardioprotective effects from lowering urinary albumin with losartan in patients with hypertension and left ventricular hypertrophy, normotensive type 2 diabetes, and advanced nephropathy and type 2 diabetes [Ibsen H et al. Hypertension 2005; Zandbergen AA et al. Diabetes Care 2007; de Zeeuw D et al. Circulation 2004].
Intriguingly, when the ACE inhibitor captopril was administered to prevent heart failure post-MI, the additional benefit of protecting the kidney, regardless of renal function, was observed, thus giving two compelling reasons to administer ACE inhibitors in these patients [Hillege HL et al. Eur Heart J 2003].
How exactly albuminuria reduction is related to CV protection is still not fully understood. But, recent data on the glycocalyx layer that is present in all small capillaries is quite interesting, as changes in glycocalyx function may explain why the kidney leaks albumin and why other vascular beds are also compromised. Thus, increased albuminuria may well reflect general vascular endothelial dysfunction [Haraldsson B et al. Physiol Rev 2008].
Defining the Cardiorenal Syndrome
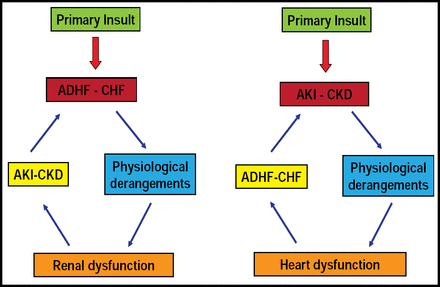
Claudio Ronco, MD, St. Bortolo Hospital, Vicenza, Italy, discussed controversies in defining the cardiorenal syndrome (CRS), for which there has been no commonly accepted definition. Instead, the term has been borrowed from other areas, with the supposition that improving CV function would also improve renal function. The condition, however, is bidirectional. Figure 2 depicts the bidirectionality of cardiorenal interactions.
Cardio-Renal Interactions.
Reproduced with permission from C. Ronco, MD.
The Acute Dialysis Quality Initiative (ADQI) was developed to provide an objective, dispassionate distillation of the literature and a description of the current state of practice to form a consensus of opinion—evidence-based when possible—on the best practices in acute renal failure and to articulate a research agenda to focus on important unanswered questions. It has held numerous conferences and has published extensively.
In 2006, the ADQI developed the RIFLE consensus classification for acute kidney injury, defining three grades of increasing severity based on changes in serum creatinine or urine output from baseline—risk (class R), injury (class I), and failure (class F)—and two outcome classes (loss and ESRD) [Bellomo R et al. Crit Care 2004]. The classification scheme can predict hospital mortality and resource use among patients in the intensive care unit [Hoste EA et al. Crit Care 2004]. A meta-analysis of 13 studies on patient mortality also suggested that even mild degrees of kidney dysfunction could increase the risk of death [Ricci Z et al. Crit Care 2006].
In 2008, the ADQI developed a definition of the CRS, defining it as a “pathophysiologic disorder of the heart and kidneys whereby acute or chronic dysfunction of 1 organ may induce acute or chronic dysfunction of the other.” The group also identified five subtypes of CRS [Ronco C et al. J Am Coll Cardiol 2008] based on pathophysiology, time frame, and nature of concomitant cardiac and renal dysfunction:
-
Type 1: An abrupt worsening of cardiac function (eg, acute cardiogenic shock or decompensated CHF), leading to acute kidney injury
-
Type 2 : Chronic abnormalities in cardiac function (eg, chronic CHF) that cause progressive CKD
-
Type 3: An abrupt worsening of renal function (eg, acute kidney ischemia or glomerulonephritis) that causes acute cardiac dysfunction (eg, heart failure, arrhythmia, ischemia)
-
Type 4: A state of CKD (eg, chronic glomerular disease) that contributes to decreased cardiac function, cardiac hypertrophy, and/or increased risk of adverse CV events
-
Type 5: A systemic condition (eg, sepsis) that causes both cardiac and renal dysfunction
While numerous biomarkers are available that can lead to an early diagnosis of CRS and therapeutic interventions that could reduce the risk of or prevent progression, clinicians remain unaware of the possibility of prevention through early intervention; the importance of monitoring biomarkers, like creatinine levels and GFR; and the implications of outcomes in this condition.
- © 2010 MD Conference Express