Summary
This article presents an analysis from the Anglo-Scandinavian Cardiac Outcomes [ASCOT] Trial database, showing that screening for high-sensitivity C-reactive protein (hsCRP) only minimally improved risk assessment in middle-aged patients with traditional cardiovascular disease risk factors.
- Prevention & Screening Clinical Trials
- Lipid Disorders
- Hypertensive Disease
Peter S. Sever, MD, Imperial College, London, UK, presented an analysis from the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT) database, showing that screening for high-sensitivity C-reactive protein (hsCRP) only minimally improved risk assessment in middle-aged patients with traditional cardiovascular disease risk factors.
This retrospective, nested, case-control study explored the relationship between hsCRP prior to and during treatment with statins and their association with cardiovascular (CV) events. ASCOT randomized 19,342 hypertensive adults aged 40–79 years with no prior CHD but with 3 or more additional CV risk factors to either a calcium channel blocker (amlodipine) or beta-blocker (atenolol) (ASCOT blood pressure-lowering arm) [Dahlöf B et al. Lancet 2005]. Patients (n=10,305) with total cholesterol ≤6.5 mmol/L (250 mg/dL) were further randomized to atorvastatin (10 mg) or placebo (ASCOT lipid-lowering arm) [Sever PS et al. Lancet 2003].
For the present analyses, cases were confined to those that occurred in ASCOT patients who were recruited in the UK and Ireland in whom stored blood samples for hsCRP analysis were available. Four hundred eighty-five cases (fatal coronary heart disease, nonfatal MI, coronary revascularization, fatal and nonfatal stroke) that occurred during the 5.5 years of follow-up from ASCOT were age- and sex-matched with 1367 controls from within the group. Cases were more likely to be smokers; have diabetes or increased systolic blood pressure and higher CRP, glucose, and creatinine levels; and be receiving statin therapy. Conditional logistic regression models were used to evaluate the association between CV events and LDL-cholesterol (LDL-C) and hsCRP.
There was a direct linear association between baseline CRP with CV events with an odds ratio (OR) of 1.21 (p=0.0004). Inclusion of hsCRP in the Framingham risk model did not significantly improve prediction of CV events (p=0.20).
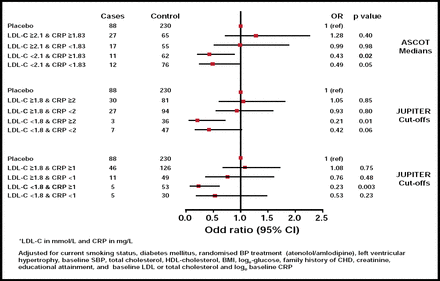
At 6 months, atorvastatin reduced median LDL-C by 40.3% and hsCRP by 27.4% (Figure 1). In subjects who were randomized to atorvastatin, lower in-trial median LDL-C (2.1 mmol/L or 77 mg/dL) was associated with a highly significant reduction in CV events (OR, 0.41; 95% CI, 0.22 to 0.75; p<0.004). In contrast, in subjects who were randomized to atorvastatin in the fully adjusted model, lower hsCRP at 6 months was not associated with CV events (OR, 0.86; 95% CI, 0.49 to 1.51; p=0.60) and, thus, was not an indicator of the magnitude of the effect of atorvastatin on CV outcome.
Risk of CV Events (CHD or Stroke) by On-Treatment (6 Month Trial) LDL-C and CRP*.
Reproduced with permission from P. Sever, MD.
In this nested case study, the addition of on-treatment hsCRP to on-treatment LDL-C did not improve prediction of statin efficacy. This modestly sized retrospective analysis does not support the hypothesis that either baseline or on-treatment hsCRP usefully improves CV risk factor prediction or provides useful information about the efficacy of statin treatment to reduce CV events beyond LDL-C reduction. These data are in contrast to those from the JUPITER trial, which studied statin therapy in a lower-risk primary prevention cohort with elevated baseline CRP, and demonstrated a significant reduction in CV endpoints. Potential explanations for the discrepant findings include the use of a lower-intensity and different statin in ASCOT (10 mg atorvastatin may not reduce CRP to the same degree as 20 mg rosuvastatin) compared with JUPITER, incomplete adjustment for baseline differences in the case-control design, and differences in study populations and outcome assessments.
- © 2010 MD Conference Express