Summary
Percutaneous closure of patent foramen ovale does not reduce the risk of recurrent stroke or transient ischemic attack of unknown origin compared with standard medical therapy alone, according to new findings from the CLOSURE I trial.
- Interventional Techniques & Devices Clinical Trials
- Cerebrovascular Disease
- Interventional Techniques & Devices
- Episodic & Paroxysmal Disorders
Percutaneous closure of patent foramen ovale (PFO), an atrial septal defect that epidemiological studies have suggested is associated with cryptogenic stroke, does not reduce the risk of recurrent stroke or transient ischemic attack (TIA) of unknown origin compared with standard medical therapy alone, according to new findings from the CLOSURE I trial.
The prospective, randomized, multicenter CLOSURE I trial included 909 patients aged 60 years or younger with a history of cryptogenic stroke or TIA and PFO that was documented by transesophageal echocardiography (TEE) within 6 months of enrollment. Patients were randomly assigned to PFO closure using the STARFlex closure device within 30 days plus 6 months of aspirin and clopidogrel, followed by an additional 18 months of aspirin (n=447) or best medical therapy (n=462), defined as aspirin, warfarin, or the combination of aspirin and warfarin for 24 months.
The composite primary endpoint included the 2-year incidence of stroke or TIA, all-cause mortality at 30 days, and neurological mortality between 31 days and 2 years. Anthony J. Furlan, MD, Case Western Reserve University School of Medicine, Cleveland, Ohio, USA, presented findings from the CLOSURE I study.
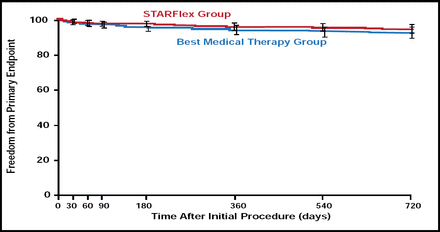
Among the patients who were randomized to the STARFlex closure device, the mean age was 46 years, 52% were male, and 38% had an atrial septal aneurysm ≥10 mm. Procedural success was achieved in 90%. In an intent-to-treat analysis, 5.9% of patients in the PFO closure group and 7.7% of those who were treated with medical therapy alone reached the primary endpoint (p=0.30; Figure 1). Stroke risk was also similar in the PFO closure and the medical therapy groups (3.1% vs 3.4%; p=0.77), as was the risk for TIA (3.3% vs 4.6%; p=0.39).
Risk of Recurrent Stroke or TIA, All-Cause Mortality, and Neurological Mortality at 2 Years.
Reproduced with permission from A. Furlan, MD.
Percutaneous PFO closure was associated with significantly more major vascular complications than medical therapy (3.2% versus 0.0%; p<0.001), as well as an increased risk of atrial fibrillation (5.7% versus 0.7%; p<0.001). Most cases of atrial fibrillation in the device closure group (60%) were periprocedural. Patients in the PFO closure group also showed a trend toward increased major bleeding (2.6% vs 1.1%; p=0.11) but experienced no increase in the risk of nonendpoint deaths (0.5% vs 0.7%) or other serious adverse events (16.9% vs 16.6%).
Given the high procedural success rate, the lack of benefit with PFO closure was not due to device failure. Thrombus formation was observed by TEE in 4 patients (1.0%), including 2 patients with a recurrent stroke on Days 4 and 52, respectively. The majority of patients maintained effective PFO closure, defined as no residual leaks by TEE at 6 months (86.1%), 12 months (86.4%), and 24 months (86.7%). Furthermore, there were no recurrent strokes or TIA in any of the patients with residual leaks. Finally, within the medical treatment group, there was no difference in the primary endpoint between aspirin alone and warfarin alone.
Among patients in the CLOSURE I trial who experienced recurrent stroke or TIA during follow-up, approximately 80% had an alternative explanation other than paradoxical embolism, Dr. Furlan said. These findings suggest that cryptogenic stroke and TIA include multiple etiologies other than PFO that are not adequately addressed with PFO closure or current medical therapy.
Although the CLOSURE I trial showed no significant improvement with PFO closure over medical therapy alone, PFO closure may be beneficial in better-defined patient subgroups, Dr. Furlan said. Ongoing trials, including the Patent Foramen Ovale Closure or Anticoagulants Versus Antiplatelet Therapy to Prevent Stroke Recurrence (CLOSE) and Patent Foramen Ovale and Cryptogenic Embolism (PC) trials, are examining the role of PFO closure in other patient groups.
- © 2010 MD Conference Express