Summary
Doubling the dose of clopidogrel in patients with high residual platelet activity after percutaneous intervention has no significant effect on cardiovascular outcomes, according to the neutral results of the Gauging Responsiveness with A VerifyNow Assay—Impact on Thrombosis And Safety [GRAVITAS; NCT00645918] trial.
- Interventional Techniques & Devices Clinical Trials
- Thrombotic Disorders
Doubling the dose of clopidogrel in patients with high residual platelet activity after percutaneous intervention (PCI) has no significant effect on cardiovascular (CV) outcomes. The neutral results of the Gauging Responsiveness with A VerifyNow Assay—Impact on Thrombosis And Safety (GRAVITAS; NCT00645918) trial were presented by Matthew Price, MD, Scripps Clinic, La Jolla, California, USA.
The trial was designed to test the hypothesis that high-dose clopidogrel for 6 months would be superior to standard-dose clopidogrel in preventing adverse CV events in patients with high residual platelet reactivity after PCI. At least 7 studies, involving more than 3000 patients, have found that high residual (on-clopidogrel) platelet reactivity, as measured by the VerifyNow P2Y12 test, is associated with poor clinical outcomes after PCI [Price MJ et al. Eur Heart J 2008; Campo G et al. J Am Coll Cardiol 2010; Marcucci R et al. Circulation 2009; Mangiacapra F et al. J Am Coll Cardiol Intv 2010; Patti G et al. J Am Coll Cardiol 2008; Migliorini A et al. Circulation 2009; Bonello L et al. J Am Coll Cardiol 2010].
While some clinicians have already begun to treat patients with residual platelet reactivity with higher doses (>300-mg load, >75-mg daily maintenance) of clopidogrel, this was the first large, randomized, clinical trial to test a treatment strategy in such a patient population.
The trial involved 5429 subjects who received the standard clopidogrel regimen around the time of the PCI procedure. Their platelet function was evaluated with the VerifyNow P2Y12 test 12 to 24 hours after PCI. Of them, 2214 (41%) had high residual platelet reactivity (platelet reactivity units [PRU] >230) and were randomized to continue on the 75-mg standard clopidogrel dose or to receive another 600-mg loading dose and a higher maintenance dose of 150 mg daily. Follow-up VerifyNow assays, the results of which were not available to the treating physician, were performed at 30 days and 6 months to assess the effect of the intervention on platelet reactivity. All participants also received daily low-dose aspirin.
The primary efficacy endpoint was CV death, nonfatal myocardial infarction (MI), or stent thrombosis at 6 months. The key safety endpoint was moderate or severe bleeding at 6 months.
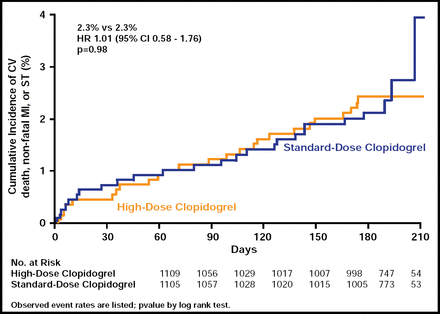
Most enrolled patients were at relatively low risk at baseline, with 84% having stable coronary artery disease or low-risk unstable angina. Results showed no significant differences between the 6-month rate of CV death, MI, or stent thrombosis, which was 2.3% for both groups (HR, 1.01; 95% CI, 0.58 to 1.76; p=0.98; Figure 1). Rates of bleeding, whether moderate or severe, were also similar in both groups (1.4% vs 2.3%, high dose and standard dose respectively; p=0.10).
Primary Endpoint: CV Death, MI, Stent Thrombosis.
Reproduced with permission from M. Price, MD.
Persistently high PRU levels (> 230) were significantly more common in the standard-dose group at 30 days (62% vs 40%; p<0.001), although achieving a lower PRU with higher-dose clopidogrel did not translate into improved clinical outcomes. One possible explanation is that the magnitude of the effect on platelet reactivity was not sufficient to demonstrate a clinically significant difference between these two dosing strategies of clopidogrel.
Dr. Price noted that high-dose clopidogrel was safe and that future trials should investigate more potent antiplatelet agents, focus on different populations, and test different treatment strategies—eg, treating to a specific PRU target rather than basing treatment upon a single post-PCI assessment of platelet function.
- © 2010 MD Conference Express