Summary
In the EMPHASIS-HF trial, the addition of eplerenone to evidence-based therapy improved survival rates for patients with mildly symptomatic systolic heart failure.
- Heart Failure Clinical Trials
The addition of eplerenone to optimal medical therapy has been shown to reduce morbidity and mortality among patients with acute myocardial infarction that is complicated by left ventricular dysfunction and NYHA Classs II heart failure (HF) [Pitt B et al. N Engl J Med 2003; Pitt B et al. N Engl J Med 1999]. In a late-breaking clinical trial that was presented by Faiez Zannad, MD, PhD, University of Nancy, Nancy, France, the addition of eplerenone to evidence-based therapy improved survival rates for patients with mildly symptomatic systolic HF.
The Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure (EMPHASIS-HF; NCT00232180) was designed to evaluate the effect of eplerenone, a selective aldosterone antagonist, on mortality and morbidity in patients with NYHA class II chronic systolic HF. The study comprised 2737 subjects (mean age 68 years; more than 75% men) with an ejection fraction ≤30% and an eGFR <30 ml/min/1.73 m2. Subjects were randomly assigned to receive either eplerenone (n=1364) or placebo (n=1373) in addition to standard HF therapy and were followed for a median of 21 months. The dose of eplerenone was adjusted from 25 mg every day to 50 mg daily, depending on serum potassium, which, along with renal function, was monitored every 4 months. The primary endpoint was a composite of time to cardiovascular (CV) death or first hospital admission for worsening HF, whichever occurred first.
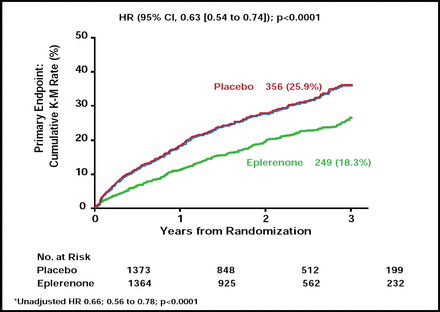
Due to the overwhelming benefit in the eplerenone arm, the trial was stopped early. Incidence of the primary outcome of of CV death or hospitalization occurred in 18.3% of patients treated with eplerenone versus 25.9% who received placebo (HR, 0.63; 95% CI, 0.54 to 0.74; p<0.0001; Figure 1). The number that was needed to treat to prevent one patient from experiencing the primary endpoint per year of follow-up was 19. The effect of eplerenone on the primary outcome was consistent across all prespecified subgroups.
Primary Endpoint CV Death or Hospitalization for HF.
Copyright © 2010 Massachusetts Medical Society. All rights reserved.
Mortality from any cause was reduced by 24% with eplerenone (12.5%) versus placebo (15.5%; p=0.0081). CV death and death from worsening HF were significantly (p=0.01 and p=0.05, respectively) reduced with the addition of eplerenone. In addition, eplerenone reduced the rate of hospitalization from any cause by 30% (p<0.001) and the rate of hospitalization for HF by 12% (p<0.001).
Hyperkalemia is a known side effect of aldosterone antagonists, whose use requires routine potassium and renal function assessments. Patients who were treated with eplerenone had significantly (8.0% vs 3.7%; incidence of hypokalemia. There were no significant differences regarding drug withdrawal due to adverse events, hospitalization for worsening renal failure, or hyperkalemia between the eplerenone and placebo treatment groups.
Findings from the EMPHASIS-HF trial in patients with class II HF expanded the results of the EPHESUS trial, which showed a benefit of eplerenone over placebo in post-MI patients with NYHA class III/IV symptoms and significant LV dysfunction. “We believe that the robustness of these findings, in conjunction with the consistent results from the earlier RALES and EPHESUS trials, provides compelling evidence to change medical practice,” concluded Prof. Zannad. Studies to examine the impact of eplerenone in high-risk, untargeted patients are planned.
This study was published simultaneously in the New England Journal of Medicine. Zannad F et al. N Engl J Med 2010.
- © 2010 MD Conference Express