Summary
New-onset diabetes after transplantation (NODAT) is recognized as one of the major medical complications of solid-organ transplantation. A spectrum of problems is attributed to NODAT, including impaired graft function, infectious complications, patient inconvenience, and increased costs, as well as patient mortality [Woodward RS et al. Am J Transplant 2003].
- Prevention & Screening
- Diabetes Mellitus
- Hyperglycemia/Hypoglycemia
New-onset diabetes after transplantation (NODAT) is recognized as one of the major medical complications of solid-organ transplantation. A spectrum of problems is attributed to NODAT, including impaired graft function, infectious complications, patient inconvenience, and increased costs, as well as patient mortality [Woodward RS et al. Am J Transplant 2003].
However, NODAT is not the same as diabetes mellitus. It has a different pathophysiology and disparate risk factors and treatment options and is associated with a greater risk of diabetic complications [Burroughs TE et al. Transplantation 2007].
Given the considerable morbidity and mortality of NODAT, efforts are underway to identify risk factors and preventive approaches. However, there is no clinically validated tool or questionnaire for predicting the risk of developing NODAT.
Two of the strongest, nonmodifiable risk factors are age and ethnicity, particularly African-Caribbeans and Hispanics. Others include human leukocyte antigen (HLA) system mismatches in kidney transplantation, with certain HLA types serving as independent predictors for the development of NODAT. There is also emerging interest in genetic susceptibilities, and some data suggest that deficiencies in the innate immune system may be related to an increased risk [Sharif A et al. Nat Rev Nephrol 2010].
Modifiable risk factors for the development of NODAT include elevated pretransplantation fasting glucose, which is associated with an increased risk 1-year posttransplant and hyperglycemia in the immediate postoperative period, even transient hyperglycemia [Chakkera HA et al. Clin J Am Soc Nephrol 2010; Chakkera HA et al. Clin J Am Soc Nephrol 2009]. The issue of transient hyperglycemia is important, because 66% of nondiabetics are discharged on insulin.
The use of an oral glucose tolerance test (OGTT) is beneficial for pretransplant risk stratification, since impaired glucose tolerance pretransplantation is predictive for posttransplant hyperglycemia [Bergrem HA et al. Nephrol Dial Transplant 2010]. The use of the OGTT is also important for predicting the risk of NODAT posttransplant, as evidence suggests that this measurement can identify more patients with NODAT than fasting glucose tests alone [Delgado P et al. Clin J Am Soc Nephrol 2008; Sharif A et al. Transplantation 2006].
While there are advantages that are associated with HbA1C use in the transplantation setting, there are also caveats that are associated with its use, including the lack of validation in the transplantation setting, the influence of posttransplantation anemia, and the myelotoxicity of immunosuppressants, all of which can affect accuracy [Sharif A et al. J Am Soc Nephrol 2010].
Immunosuppressive agents represent another modifiable risk factor for NODAT. While many are diabetogenic, some, including mycophenolate mofetil, azathioprine, and biological induction agents, are not [Sharif A et al. Nat Rev Nephrol 2010]. Other modifiable risk factors include obesity; biochemical abnormalities, such as posttransplantation hypomagnesemia, uric acid, vitamin D deficiency, and renal or renal graft function; and the use of antihypertensives [Armstrong KA et al. Nephrology 2005; Van Laecke A et al. Am J Transplant 2009; Sharif A, et al. Am J Transplant 2009; Hjelmesaeth J et al. Diabetes Care 2001].
Studies are underway to identify the potential protective effects of pharmacological prophylaxis with sitagliptin to prevent NODAT following kidney transplantation.
Treating NODAT
Most of the current data on NODAT focus on immunosuppressant therapies and prevention strategies. However, there are little data on the optimal treatment for this condition. Current goals for therapy are based on American Diabetes Association guidelines for nontransplant diabetics. They include target HbA1C of <7% following transplant, premeal glucose of 90–130 mg/dL, postprandial glucose of <180 mg/dL, blood pressure of <130/80 mm Hg, low-density lipoprotein (LDL) cholesterol of <100 mg/dL, high-density lipoprotein (HDL) cholesterol of >40 mg/dL, and triglycerides of <150 mg/dL [Reynolds LR et al. Diabetes Care 2001].
Lisa Tannock, MD, University of Kentucky, Lexington, Kentucky, USA, discussed treatment options for NODAT. Dr. Tannock's group does not utilize oral antidiabetic agents for hospitalized patients—only insulin. Patients who receive intravenous (IV) nutrition are managed on IV insulin, while those who are able to eat receive basal/bolus insulin.
In the outpatient setting, patients may be managed with either insulin or oral agents. Patients with blood glucose levels >300 mg/dL (16 mmol/L) begin on insulin, with the regimen dependent on the patient. The two algorithms that are used are either 10 units twice daily titrated by 2 units every 1 to 2 days or a body weight dosing formula. In extremely obese patients, body weight dosing is used. Patients with more moderate hyperglycemia receive education and evaluation, possibly leading to adjustment of their immunosuppressive agents and insulin or oral therapies, as needed.
When using oral antidiabetic agents, metformin is affordable, effective, and well tolerated, with no associated weight gain or hyperglycemia. Although there are little data on its use in the transplant population, it appears safe in patients who do not have renal insufficiency, significant liver disease, or congestive heart failure.
The thiazolidinediones (TZD) appear to be safe for use in patients with chronic kidney disease; however, they should be used cautiously in patients with liver dysfunction. It is important to note that cardiovascular risk that is associated with TZD use is unclear, and TZD therapy may increase the risk of fractures and may result in weight gain and edema.
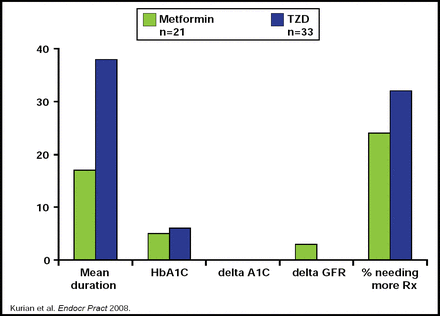
A retrospective study that addressed the duration and efficacy of TZDs versus metformin in transplant patients found that while HbA1C levels were similar between the two groups, patients who were on TZDs had a greater duration of glycemic control (Figure 1). However, they ultimately needed further treatment as the study progressed. Additionally, metformin demonstrated a greater effect on glomerular filtration rate (GFR) levels, raising concerns about its use in patients with renal disease [Kurian B et al. Endocr Pract 2008].
Metformin Versus TZD in Renal Transplant Recipients.
Reproduced with permission from L. Tannock, MD.
Secretagogues are also effective in NODAT patients, but these compounds require more frequent dosing and are metabolized via CYP3A4, raising concerns regarding drug interactions. There are little data on other oral hyperglycemic options in this population.
In conclusion, it is clear that more research is needed as to the best approach to prevent and treat NODAT. Greater collaborations between diabetologists and transplant physicians are required to ensure the delivery of optimum care.
- © 2010 MD Conference Express