Summary
This article discusses both the linkages between glucose and bone metabolism that are actively being researched, as well as the effects of glucose-lowering agents on bones.
- Metabolic Bone Disease
- Diabetes Mellitus
The Linkages Between Glucose and Bone Metabolism are an Active Area of Research
Bone itself has an effect on glucose metabolism through osteocalcin, a hormone that enhances insulin secretion and sensitivity, increasing β-cell mass and energy expenditure. Esp, an osteoblast-specific gene, inhibits osteocalcin function by favoring its carboxylation into the inactive form, blunting glucose handling [Lee NK et al. Cell 2007].
Insulin receptors in osteoblasts are substrates of Esp. In a series of animal studies, it was determined that inactivating insulin receptors in osteoblasts affects whole-body glucose metabolism. Indeed, insulin signaling in osteoblasts enhances bone resorption, and the acid pH that is generated by bone resorption activates osteocalcin via its decarboxylation. This regulatory mechanism controls whole-body glucose homeostasis in mice and humans [Ferron et al. Cell 2010].
The associations between glucose and bone metabolism likely contribute to the increased risk of bone fractures that is associated with both type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM) [Vestergaard P. Osteoporosis Int 2007; Vestergaard P et al. Diabetologia 2005]. However, the mechanisms of this increased risk appear to differ somewhat from the risk factors for osteoporosis and fracture in the general population.
For instance, patients with T1DM have far less bone density than would be expected in their bones and hips, while patients with T2DM have greater bone density than would be expected for their age [Vestergaard P. Osteoporosis Int 2007]. Yet, rather than the 20% to 25% reduced fracture risk that might be expected in T2DM, the actual risk is 70% higher. Alternatively, rather than the 42% increased risk of fracture that might be expected in patients with T1DM, the actual risk is increased nearly 6-fold [Janghorbani M et al. Am J Epidemiol 2007]. This suggests that bone quality, not just bone density, is related to the fracture risk in diabetes. Indeed, diabetes is a disease of low bone turnover [Hamada Y et al. Bone 2007].
Direct effects of diabetes that contribute to the higher incidence of fracture and lower bone turnover include hyperglycemia, with marked declines in serum markers of bone resorption and bone turnover occurring within 2 hours of ingesting glucose during an oral glucose tolerance test (OGTT) [Clowes JA et al. J Clin Endocrinol Metab 2003].
Increased calcium excretion (hypercalciuria) is observed early in the disease in patients with T1DM. These levels eventually moderate and return to normal, which could be related to improved control of hyperglycemia [McNair P et al. Acta Endocrinol (Copenh) 1979]. This is also reflected in fracture risk, which appears to be higher early in the disease [Vestergaard P et al. Calcif Tissue Int 2009]. The reduction in calcium levels typically results in higher levels of parathyroid (PTH) hormone. However, although PTH levels rise in patients with diabetes, the peak is far lower than in individuals without diabetes [Schwarz P et al. Acta Endocrinol 1992].
High blood glucose levels also affect the bone matrix, leading to the formation of advanced glycated end-products (AGEs), such as pentosidine, which results in lower bone biomechanical competence [Saito M et al. Osteoporosis Int 2010].
More indirect effects of diabetes on bone are related to high blood pressure. Studies in nondiabetic patients find results in greater urinary calcium excretion and bone loss; neuropathy, likely related to reduced physical activity; and macrovascular complications that reduce blood flow to bone [Cappuccio FP et al. Lancet 1999; Rix M et al. Diabetes Care 1999; Vogt MT et al. J Bone Miner Res 1997]. Body mass index (BMI) is important in both diabetic and nondiabetic patients. The leaner body mass of patients with T1DM may help explain their lower bone density compared with those with T2DM, who tend to be heavier [Vestergaard P. Osteoporosis Int 2007]. Finally, increased PPAR-γ levels that result from chronic hyperglycemia, which suppresses osteoblast differentiation, promotes an adipocyte-like phenotype that results in less bone formation [Botolin S et al. J Cell Biochem 2006].
The Effects of Glucose-Lowering Agents on Bone
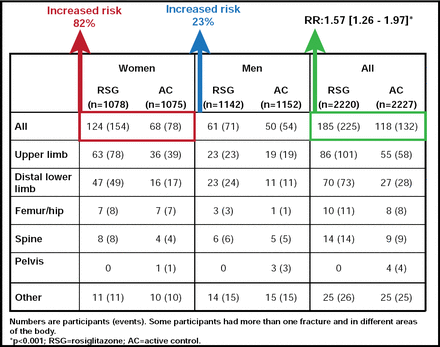
In addition to the mechanisms described above, certain glucose-lowering drugs can impact bone. Among the most studied are the thiazolidinediones rosiglitazone and pioglitazone. Both are independently associated with an increased risk of bone fracture in men and women with T2DM, with a doubling of the risk in women (Figure 1) [Kahn SE et al. Diabetes Care 2008; Aubert RE et al. Diabetes Obes Metab 2010; Home PD et al. Lancet 2009].
Rosiglitazone and Bone Fracture Risk in Women with T2DM.
Reprinted from The Lancet. Volume 373, Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicenter, randomized, open-label trial, RECORD Study Group, pages 2125–2135, Copyright 2009, with permission from Elsevier.
This increased risk may be related to increased osteoclast activity rather than decreased osteoblast activity [Zinman B et al. Lancet 2010]. Animal and human studies suggest that these drugs may suppress osteoblast differentiation, decreasing osteoblastogenesis in human mesenchymal stem cells [Ali AA et al. Endocrinology 2005; Benvenuti S et al. J Endocrinol Invest 2007].
While a large case-control study of 2000 patients with T2DM who were followed for 4 years found a significant increase in bone fracture in patients who were using insulin, there was no reduction in bone mineral density [Monami M et al. Diabetes Care 2008; Stolk RP et al. Bone 1996]. The mechanism may be related more to the higher rate of hypoglycemia that occurs with insulin therapy, increasing the risk of falls [Schwartz AV et al. Diabetes Care 2002; Adami S. Curr Med Res Opin 2009].
Although hypoglycemia can also be induced by secretagogues, the risk is much lower than that observed with insulin, which may explain why insulin secretagogues are not associated with an increased risk of bone fracture [Vestergaard P et al. Diabetologia 2005; Monami M et al. Diabetes Care 2008]. Metformin also appears to have neutral effects on bone [Vestergaard P et al. Diabetologia 2005; Monami M et al. Diabetes Care 2008].
Meanwhile, the incretin-based therapies may exert some favorable effects on bone metabolism. Bone has receptors for glucagon-like peptide-1 (GLP-1). In animal models, GLP-1 receptor knockout mice exhibited higher osteoblast activity than wild-type mice, while even short-term treatment with the GLP-1 agonist exenatide stimulated the deposition of new bone in animal models of T2DM and insulin resistance [Nuche-Berenguer B et al. Regul Pept 2010]. Responsible mechanisms may be the ability of GLP-1 to prevent the differentiation of mesenchymal stem cells into adipocytes or stimulate thyroid C-cells, in turn stimulating increased calcitonin release and C-cell proliferation [Knudsen BL et al. Endocrinology 2010; Sanz C et al. Am J Physiol Endocrinol Metab 2010].
The dipeptidyl peptidase-4 (DPP4) inhibitors could affect bone both by increasing circulating GLP-1 levels and by increasing gastric inhibitory polypeptide, thus having a favorable effect on osteogenesis while decreasing bone resorption [Baggio LL et al. Gastroenterology 2007].
In conclusion, the low bone turnover in patients with diabetes may have implications for the treatment of osteoporosis in this population. The traditional therapy for osteoporosis is antiresorptive agents. However, this may contribute to further reductions in bone turnover. In addition, there seems to be a discrepancy between bone mineral density, as measured by dual-energy x-ray absorptiometry (DXA) scans, and bone biomechanical competence, especially in T2DM. Thus, it is unclear how DXA scans should be interpreted in diabetes. Finally, bone fractures should be considered among the treatment outcomes when choosing antidiabetic medications.
- © 2010 MD Conference Express