Summary
In patients with diabetes, vascular conditions are responsible for the majority of morbidity, mortality, and cost that are attributed to the disease [Centers for Disease Control and Prevention. National Diabetes Fact Sheet. 2007]. The Liraglutide Effect and Action in Diabetes [LEAD; NCT00700817] trials are designed to evaluate the efficacy and safety of glucagon-like peptide-1 agonist compared with existing antidiabetic therapies.
- Diabetes & Endocrinology Clinical Trials
- Hyperglycemia/Hypoglycemia
- Diabetes Mellitus
In patients with diabetes, vascular conditions are responsible for the majority of morbidity, mortality, and cost that are attributed to the disease [Centers for Disease Control and Prevention National Diabetes Fact Sheet. 2007]. Yet, a significant number of people with type 2 diabetes mellitus (T2DM), the most prevalent form of the disease, do not maintain good glycemic control, in part due to inadequate efficacy and/or side effects that are associated with currently available therapies. The Liraglutide Effect and Action in Diabetes (LEAD; NCT00700817) trials are designed to evaluate the efficacy and safety of glucagon-like peptide-1 (GLP-1) agonist compared with existing antidiabetic therapies.
The latest results from LEAD were presented by Richard Pratley, MD, University of Vermont College of Medicine, Burlington, Vermont, USA. In this active-comparator, parallel-group, open-label trial, 658 participants with inadequate glycemic control who were on metformin were randomized to receive 1.2 mg (n=225) or 1.8 mg (n=221) of subcutaneous liraglutide once daily or 100 mg of oral sitagliptin (n=219) once daily, in addition to their baseline metformin dose, for 26 weeks, with the option of continuing into a 12-month follow-up phase. The primary efficacy endpoint was change in HbA1C from baseline to Week 26. Secondary endpoints included HbA1C targets of <7% or <6.5%; fasting plasma glucose; postprandial plasma glucose; body weight; β-cell function; fasting lipid profile; cardiovascular risk markers, blood pressure, heart rate, physical measurements, and treatment satisfaction; and a composite endpoint of the percentage of participants with HbA1C <7% with no hypoglycemia and a weight change of 0 kg or less at baseline and Week 26.
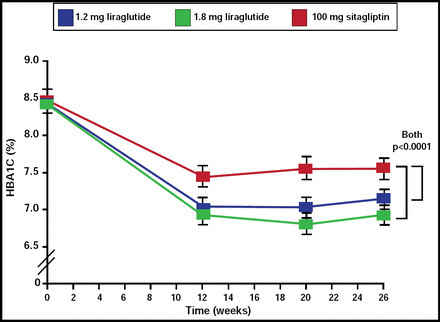
The liraglutide cohorts demonstrated superior HbA1C reductions compared with the sitagliptin group (p<0.0001; Figure 1). After 26 weeks, the liraglutide group demonstrated mean decreases in HbA1C from baseline of 1.50% (95% CI, −1.63 to −1.37) for the 1.8-mg dose and 1.24% (−1.37 to −1.11) for the 1.2-mg dose, while the sitagliptin group experienced a 0.90% reduction (−1.03 to −0.77; p<0.0001 for both).
Differences in Primary Efficacy Endpoint (Change in HbA1C).
Reprinted from The Lancet. Volume 375, Issue 9724, Pratley RE et al, Liraglutide versus sitagliptin for patients with type 2 diabetes who did not have adequate glycemic control with metformin: a 26-week, randomized, parallel-group, open-label trial, pages 1447–1456, Copyright 2010, with permission from Elsevier.
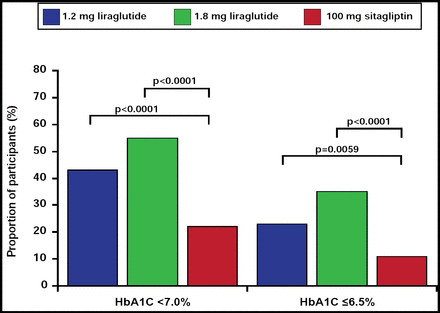
In addition, a greater proportion of patients in the liraglutide groups achieved the target HbA1C goal of <7.0% or ≤6.5% (Figure 2; achievement of HbA1C <6.5% OR, 4.25; 95% CI, 2.55 to 7.08 in the liraglutide 1.8-mg group and OR, 2.11; 95% CI, 1.24 to 3.59 in the 1.2-mg group; achievement of HbA1C <7.0% OR, 4.50; 95% CI, 2.90 to 6.97 in the liraglutide 1.8-mg group and OR, 2.75; 95% CI, 1.78 to 4.25 in the 1.2-mg group) compared with the sitagliptin cohort. The liraglutide groups also experienced significantly greater mean decreases in fasting plasma glucose (p<0.0001 for both).
Proportion of Participants Achieving HbA1C Target Values.
Reprinted from The Lancet. Volume 375, Issue 9724, Pratley RE et al, Liraglutide versus sitagliptin for patients with type 2 diabetes who did not have adequate glycemic control with metformin: a 26-week, randomized, parallel-group, open-label trial, pages 1447–1456, Copyright 2010, with permission from Elsevier.
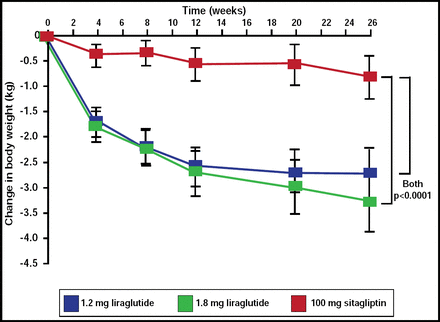
Of particular interest was the change in body weight between the liraglutide and sitagliptin cohorts (Figure 3). The liraglutide 1.8-mg group lost a mean of 3.38 kg, and the 1.2-mg group lost a mean of 2.86 kg, while the sitagliptin group lost a mean of 0.96 kg (p<0.0001 for both).
Change in Body Weight.
Reprinted from The Lancet. Volume 375, Issue 9724, Pratley RE et al, Liraglutide versus sitagliptin for patients with type 2 diabetes who did not have adequate glycemic control with metformin: a 26-week, randomized, parallel-group, open-label trial, pages 1447–1456, Copyright 2010, with permission from Elsevier.
Despite the daily injection that was required with liraglutide, there was no difference in the perceived convenience of the two compounds between participants.
More treatment-emergent adverse events occurred with liraglutide than sitagliptin, although serious events occurred in 3% or fewer of the participants. The most common adverse events were gastrointestinal problems, particularly nausea, which were higher in the liraglutide groups, and infections and infestations, which occurred equally between all groups. The mean duration of nausea in the liraglutide 1.2-mg group was 13 days versus 8 days among the 1.8-mg group and 26 days among the sitagliptin group.
One major hypoglycemic episode occurred in a participant in the 1.8 mg liraglutide cohort. However, no seizures or coma resulted. Similar proportions of patients in each group experienced mild hypoglycemia.
In conclusion, liraglutide 1.2 mg and 1.8 mg provided superior glycemic control and body weight loss compared with sitagliptin 100 mg with no increase in hypoglycemia, although a greater proportion of patients who received liraglutide reported transient nausea that lasted a mean of 8 or 13 days, depending upon the dose.
- © 2010 MD Conference Express