Summary
While several major disturbances contribute to the pathogenesis of congestive heart failure, perhaps the most difficult to understand and the most complex is insulin resistance.
- Cardiometabolic Disorder
- Heart Failure
- Inflammatory Disease
While several major disturbances contribute to the pathogenesis of congestive heart failure (CHF), perhaps the most difficult to understand and the most complex is insulin resistance.
In a study by Ingelsson and colleagues, insulin resistance was found to predict CHF incidence independently of other factors, including diabetes, suggesting that the longstanding association between obesity and CHF may be mediated by insulin resistance [Ingelsson E et al. JAMA 2005]. Meanwhile, a study by Doehner and colleagues found that lower insulin sensitivity was associated with higher mortality in patients with CHF, regardless of weight, suggesting that impaired insulin sensitivity might contribute to CHF disease progression [Doehner W et al. J Am Coll Cardiol 2005].
However, the question of whether insulin resistance is a mediator or a marker of heart failure remains.
Insulin resistance is defined as “the diminished ability of cells to respond to the action of insulin in transporting glucose from the bloodstream into muscle.” The essence of insulin resistance and its metabolic derangements are an excess in fuel supply (glucose) and a decrease in fuel oxidation.
In diabetes, the fuel supply is primarily dysregulated through increased lipolysis, hepatic lipogenesis, hepatic gluconeogenesis, and hepatic glucose production. These processes affect the entire body, as well as the heart, through increased blood glucose and free fatty acid levels.
The heart itself is somewhat insulin-resistant, even in healthy individuals, possibly to protect itself from being flooded with fuel. The evidence for this theory comes from biochemical, physiological, and immunological scientific findings, as well as anecdotal evidence that is found in the so-called “obesity paradox,” in which some individuals who are quite obese do not develop cardiovascular abnormalities [Kolka CM et al. Diabetes 2010; Jagasia D et al. Circulation 2001; Srinivasan M et al. J Am Coll Cardiol 2005; Hotamisligil GS et al. J Clin Invest 1995].
In one study that supports this theory, an insulin infusion in patients with type 2 diabetes mellitus (T2DM) decreased sinus blood flow rather than increasing perfusion, as typically occurs in normal, nondiabetic individuals [Jagasia D et al. Circulation 2001]. In another study, myocardial blood flow was measured at rest and during adenosine stress under normal metabolic conditions and then during a hyperinsulinemic euglycemic (HE) clamp or hyperinsulinemic hyperglycemic (HH) clamp. Myocardial blood flow at rest and during adenosine administration decreased in the HH group but not in the HE group, suggesting that substrate delivery to the heart had already decreased at the capillary level [Srinivasan M et al. J Am Coll Cardiol 2005].
Thus, a dysregulated fuel supply impairs cardiac function.
Yet, it is also known that impaired fatty acid oxidation significantly affects cardiovascular (CV) health, resulting in an extremely lipophilic heart muscle with contractile dysfunction in both animal and human models [Haemmerle G et al. Science 2006; Chiu HC et al. J Clin Invest 2001; Sharma S et al. FASEB J 2004]. Approximately one-third of patients with nonischemic CHF who are referred for cardiac transplantation demonstrate lipotoxicity, particularly if they are obese and/or diabetic [Sharma S et al. FASEB J 2004].
CHF also involves impaired fatty acid oxidation. This is important, since obesity and diabetes increase fatty acid delivery. The resulting intramyocardial lipid overload results in reactive oxygen species, diacylglycerol production, and protein kinase C activity, leading to insulin resistance; ceramide accumulation, which triggers apoptosis; and altered gene expression, resulting in contractile dysfunction [Sharma S et al. FASEB J 2004].
In other words, the impaired fatty acid oxidation appears to occur first, followed by insulin resistance and the resulting glucotoxicity. Thus, it appears that insulin resistance is a marker, not a mediator, of premature death and disability from CHD.
Diabetic Cardiomyopathy: A Dynamic Phenotype
There appear to be two distinct diabetic cardiomyopathy phenotypes: congestive dilated cardiomyopathy with left ventricular (LV) hypertrophy and fibrosis; and a more restrictive cardiomyopathy with normal ejection fraction (EF), a small LV cavity, delayed diastolic relaxation, and increased filling pressure. There may also be an increased ratio of early transmitral velocity to tissue diastolic velocity in this latter type [Marwick. Diabetic Cardiomyopathy In: Crawford MH, DiMarco JP, Paulus WJ, eds. Cardiology, 3rd Edition. Philadelphia: Elsevier; 2009].
While the two CHF types are distinct, there is evidence that one may evolve from the other. The concept of phenotypic evolution is not new; it was first discussed in the original description of diabetic cardiomyopathy by Zarich and colleagues in 1988, in which high diastolic LV stiffness was identified as the earliest manifestation of diabetes-induced LV dysfunction [Zarich SW et al. J Am Coll Cardiol 1988].
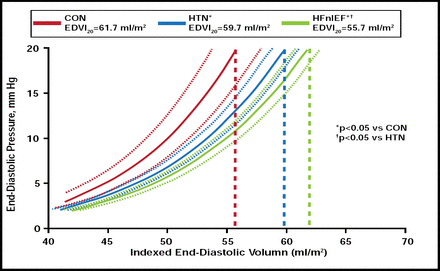
Longitudinal studies also provide evidence. In the Olmsted County cohort, Lam and colleagues found that patients with CHF and normal EF had more impaired relaxation and increased diastolic stiffness compared with patients without CV disease or those with hypertension only (Figure 1) [Lam CS et al. Circulation 2007]. This suggests that cardiac remodeling continues in the same direction, creating a smaller LV cavity with more pronounced diastolic dysfunction. Lieb and colleagues revealed similar results as part of the Framingham cohort. The greater a participant's CV risk factors were over time, the greater their increase in LV mass [Lieb W et al. Circulation 2009].
From Hypertension without HF to HF with Normal EF.
Reprinted from Circluation. Volume 115, Issue 15, Cardiac structure and ventricular-vascular function in persons with heart failure and preserved ejection fraction from Olmstead County Minnesota, CS Lam et al, pages 1982–1990, Copyright 2007, with permission from Wolters Kluwer.
Thus, it is clear that a phenotypic evolution is occurring, although they are two very distinct phenotypes and should be considered as separate entities.
Glucotoxicity or Lipotoxicity: Which is the Culprit in CHF?
Another question in diabetic cardiomyopathy is whether glucotoxicity or lipotoxicity is the “culprit” for the underlying pathogenesis.
As noted earlier, a major problem in the diabetic heart is an overabundance of substrate, whether fatty acids or glucose, and the inability to oxidize it completely. This, in turn, increases oxidative stress, decreases free fatty acid oxidation, increases rapid oxidation, and results in lipid accumulation and associated increases in nitrogenous stress. This stimulates cellular death pathways and can lead to decreases in diastolic function and increased CV morbidity and mortality.
The two processes are intertwined, with each affecting the other. For instance, increased fatty acid oxidation further impairs glycolysis. The additional glucose increases oxidative stress.
However, as noted earlier, fatty acid production increases with insulin resistance prior to any increased glucose output. Thus, it appears that the heart responds first to an increased fatty acid burden. Even when glucotoxicity occurs, the effects are relatively benign, primarily hypertrophy, which is often protective. Lipotoxicity phenotypes include premature death, LV dysfunction, and lipid accumulation, suggesting that fatty acids are a greater “culprit” than glucose [Fulop N et al. Am J Physiol Cell Physiol 2007; Zhou YT et al. Proc Natl Acad Sci USA 2000].
Indeed, there is evidence that individuals with T2DM have significantly higher myocardial triglyceride content than healthy volunteers (p<0.05) and significantly impaired diastolic function, suggesting that the presence of a lipotoxic pattern is associated with decline in diastolic function [Rijzewijk LJ et al. J Am Coll Cardiol 2008]. In addition, other evidence finds that increases in CV lipids, but not glucose, reduce myocardial energetics, particularly in people with diabetes [Scheuermann-Freestone M et al. Circulation 2003].
There also appear to be gender differences in the ability to metabolize CV lipids and glucose. Peterson and colleagues demonstrated a progressive increase in fatty acid metabolism in healthy, obese individuals, with higher fatty acid metabolism in women than men, likely due to their greater body fat content. However, while lean men exhibited greater glucose utilization, this dropped precipitously as the men gained weight, with little change in women [Peterson LR et al. JACC Cardiovasc Imaging 2008]. Other evidence that is currently in publication demonstrated that women have a greater decline in diastolic function once they develop diabetes than men, while men experience a very dramatic decline in myocardial glucose utilization with obesity, even as women experience an increase [Peterson et al. Obesity. In press]. These findings suggest that women may be more susceptible to the toxic effects of glucose on the heart and that men are more susceptible to lipotoxicity. Indeed, these findings may also help explain gender differences in response to antidiabetic drugs, particularly the insulin sensitizers and metformin [Lyons et al. Circulation. In press].
The impact of insulin resistance, glucotoxicity, and lipotoxicity on diabetic cardiomyopathy remains quite complex; further elucidation of the intertwined connections between these and other metabolic processes may provide important data for the development of new, much-needed therapeutic approaches.
- © 2010 MD Conference Express