Summary
This article discusses studies that have provided insights into the role of intravascular ultrasound guidance in optimal coronary stenting, predictors of thrombosis after successful implantation of drug-eluting stents, an the incidence and mechanisms of stent thrombosis, among other things.
- Interventional Techniques & Devices Thrombotic Disorders
Brian Ó Murchú, MD, Temple University, Philadelphia, Pennsylvania, USA, discussed studies that have provided insights into the role of intravascular ultrasound (IVUS) guidance in optimal coronary stenting. Among the first studies to identify a role for IVUS in stenting was the CRUISE substudy of the STARS trial, which suggested that IVUS guidance of stent implantation may result in more effective stent expansion compared with angiographic guidance alone. After 9 months, centers that used IVUS guidance achieved significantly larger minimal stent dimensions than centers that used angiographic guidance alone (ie, minimal lumen diameter, 2.9±0.4 vs 2.7±0.5 mm, p<0.001 by quantitative coronary angiography and minimal stent area; 7.78±1.72 vs 7.06±2.13 mm2, p<0.001 by quantitative coronary ultrasound. [Fitzgerald PJ et al. Circulation 2000]. In 2008, further evidence of a possible benefit for IVUS was provided via the results single center registry that compared the rate of definite stent thrombosis at 12 months between 884 consecutive patients who were undergoing IVUS-guided intracoronary drug-eluting stent (DES) implantation and a propensity-score matched population that was undergoing DES implantation with angiographic guidance alone. The results of this observational study indicated that IVUS guidance during DES implantation has the potential to influence treatment strategy and reduce both DES thrombosis and the need for repeat revascularization [Roy P et al. Eur Heart J 2008]. Researchers are anxiously awaiting the results of the Angiography Versus IVUS Optimisation trial (AVIO; NCT00936169), a randomized, open-label trial that aims to determine whether IVUS-guided DES implantation in complex lesions is superior to angiographically-guided implantation in improving postprocedural minimum lumen diameter.
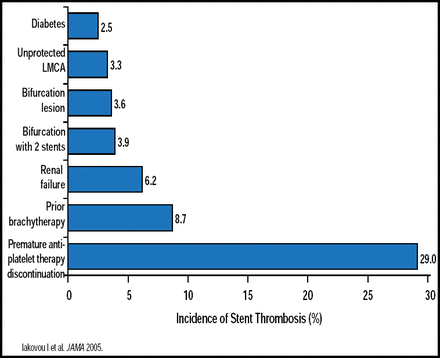
Stent thrombosis remains an important complication for both DES and bare metal stents (BMS). Alaide Chieffo, MD, San Raffaele Scientific Institute, Milan, Italy, discussed some predictors of thrombosis after successful implantation of DES (Figure 1), including dual antiplatelet therapy (DAPT)—an important component of poststent therapy whose premature discontinuation has been shown to be a significant predictor of thrombosis [Airoldi F et al. Circulation 2007; Park SJ et al. N Engl J Med 2010]. Other factors that influence the efficacy of DAPT include genotype or phenotype issues that influence a patient's ability to metabolize clopidogrel [Gurbel PA et al. Expert Opin Drug Metab Toxicol 2009; Holmes DR et al. J Am Col Cardiol 2010], as well as the overall efficacy of newer antiplatelet dugs, such as prasugrel [Wiviott SD et al. N Engl J Med 2007] and ticagrelor [Wallentin L et al. N Engl J Med 2009].
Predictors of Thrombosis After Successful Implantation of DES.
Reproduced with permission from A. Chieffo, MD.
Although the incidence of coronary artery aneurysms (CANs) after DES implantation is rare (0.2% to 2.3% of patients) [Aoki J et al. J Am Col Cardiovasc Interv 2008], they are frequently associated with adverse clinical events. Fernando Alfonso, MD, PhD, San Carlos University Hospital, Madrid, Spain, discussed the results of a study that was conducted to assess clinical, angiographic, and IVUS findings in patients who were developing a CAN after DES implantation [Alfonso F et al. J Am Coll Cardiol 2009]. The study comprised 1197 consecutive patients with late angiographic evaluation after DES implantation. CANs developed in 15 patients (1.25%; 95% CI, 0.58 to 1.93) and were more frequent when DES were implanted during acute myocardial infarction, in occluded vessels, long lesions, or residual dissections. Most patients with a CAN had an adverse prognosis (including DES thrombosis and death) that was almost always related to discontinuation of DAPT. After a mean follow-up of 399±347 days, the 1-year event-free survival was 49±14% and was related to CAN size on IVUS. While coronary angiography was able to provide an accurate diagnosis of the CAN, IVUS provided further anatomical insights, including the extent of DES malapposition, which may have major prognostic implications (Table 1).
IVUS Data of Coronary Aneursym According to Presence of DES Thrombosis.
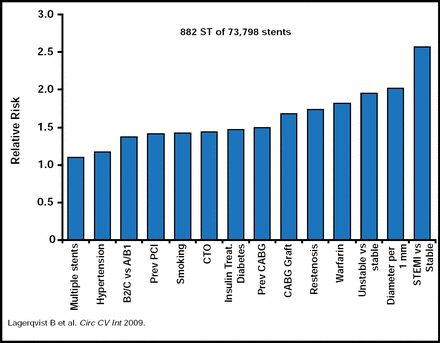
While discussing the incidence and mechanisms of stent thrombosis (ST), Stefan James, MD, Uppsala Clinical Research Center, Uppsala, Sweden, noted that it is an uncommon event, occurring in 0.5% to 1% of all patients during the first 6 months after stent implantation and then at a rate of ∼0.5% per year for DES patients thereafter. The strongest predictor of ST is the presence of a STEMI as the original indication for stenting (Figure 2) [Lagerqvist B et al. Circ Cardiovasc Int 2009]. The use of stents in off-label indications and increased platelet aggregation are known to increase the risk of ST, while more efficient platelet aggregation reduces the risk [Angiolillo DJ et al. Am J Cardiovasc Drugs 2007]. The use of DES is an independent predictor of ST only after the initial 6 months. There is also strong evidence that carriers of a reduced-function CYP2C19 allele who are treated with clopidogrel have significantly lower levels of the active metabolite of clopidogrel, diminished platelet inhibition, and a higher rate of major adverse cardiovascular events (MACEs), including stent thrombosis, than noncarriers [Mega JL et al. N Engl J Med 2009].
Independent Predictors of Stent Thrombosis in Sweden.
Reproduced with permission from S. James, MD.
Individuals who receive clopidogrel exhibit wide variability in platelet responses [Serebruany VL et al. J Am Coll Cardiol 2005; Hochholzer W et al. Circulation 2005; Trenk D et al. J Am Coll Cardiol 2008]; however, residual platelet function is a strong independent predictor of ST. Two large clinical studies are assessing the benefit of tailored antiplatelet therapy in elective PCI.
Two ongoing prospective, randomized, multicenter, double-blind, placebo-controlled trials are exploring novel strategies to reduce ischemic complications poststenting. The Gauging Responsiveness With A VerifyNow Assay-Impact On Thrombosis And Safety study (GRAVITAS; NCT00645918) is designed to assess whether tailored antiplatelet therapy for poor responders, identified based on the results of the VerifyNow P2Y12 Test, reduces MACEs (eg, heart attack, stent thrombosis) following percutaneous coronary intervention (PCI). The study population comprises ∼2800 patients with stable angina/ischemia or non-ST-elevation acute coronary syndrome who are undergoing PCI with DES. Patients with high residual platelet reactivity who are on clopidogrel therapy 12 to 24 hours post-PCI will be randomized to standard maintenance clopidogrel therapy (75 mg daily) or high-dose clopidogrel therapy (additional loading dose followed by 150 mg daily) for 6 months. A random sample of patients without high residual reactivity will be followed and treated with standard clopidogrel therapy for 6 months. The primary endpoint is the time to first occurrence of cardiovascular (CV) death, nonfatal myocardial infarction, or definite/probable stent thrombosis. The study design and rationale have been previously published [Price MJ et al. Am Heart J 2009]. Results will be reported at the American Heart Association meeting in November 2010.
Testing Platelet Reactivity In Patients Undergoing Elective Stent Placement on Clopidogrel to Guide Alternative Therapy With Prasugrel (TRIGGER-PC; NCT00910299) trial is designed to determine the efficacy of prasugrel versus clopidogrel for the reduction of adverse CV outcomes in patients with high platelet reactivity who are on clopidogrel after successful implantation of DES. The primary study outcome is time to first occurrence of heart attack or CV death. The study plans to enroll 2150 men or women with coronary artery disease and successful PCI with at least one DES. Subjects will be randomly assigned to receive a one-time 60-mg oral loading dose and a 10-mg once-daily oral maintenance dose of prasugrel for up to 6 months or clopidogrel 75 mg oral daily for up to 6 months. This study is enrolling and is not expected to complete until July 2012.
- © 2010 MD Conference Express