Summary
This article discusses clinical trials related to atrial fibrillation (AF), prevention and treatment options, and the expansion of the use of ablation in paroxysmal AF.
- Arrhythmias
Optimal Approaches
Candidate selection is an important consideration in the optimal use of ablation in patients with paroxysmal atrial fibrillation (AF). Noting the technological strides that have occurred over the past 5 years, Eric N. Prystowsky, MD, Indianapolis, Indiana, USA, discussed the results from some of the studies (at least 13 clinical studies and several meta-analyses; Table 1) that have helped to expand the use of ablation in paroxysmal AF in the time between the last Guidelines in 2006 and the 2010 update.
Clinical Studies in Ablation for Paroxsymal AF.
Relevant Meta-Analyses:
Nair GM et al. A systematic review of randomized trials comparing radiofrequency ablation with antiarrhythmic medications in patients with atrial fibrillation. J Cardiovasc Electrophysiol 2009;20:138–44.
Wilber DJ, et al. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: a randomized controlled trial. JAMA 2010;303:333–40.
“Even though 25% to 30% of patients still require multiple procedures,” said Dr. Prystowsky, “the overall success rate (which he defined as the absence of the need for antiarrhythmic drug therapy) for ablation in paroxysmal AF is very good (70% to 80%).” Based on his own experience and recent data, he suggests that ablation should be considered first-line therapy for AF in patients:
-
with very symptomatic AF who refuse antiarrhythmic drug therapy
-
in whom the only antiarrhythmic drug (AAD) choice is amiodarone
-
with brady-tachy syndrome in whom AAD can be used only with an implantable pacemaker
Ablation of chronic AF is more difficult than paroxysmal AF, because the clinical pattern of the patient often does not correspond to the anatomical background of the disease. “In clinical practice,” said Carlo Pappone, MD, Villa Maria Hospital Group, Cotignola, Italy, “we must consider many things when deciding on treatment. Important among these are the type of AF and the presence of comorbid conditions.”
Types of AF:
-
Electrical AF – usually lone paroxysmal AF with simple substrates and no fibrosis but having discernable electrical signals all over the left atrium (LA) during AF.
-
Anatomical AF – often coincident with long-standing permanent AF and having complex substrates and extensive fibrosis with the absence of clear electrical activity in the majority of the LA during AF.
-
Mixed AF – the most frequent form of “chronic” AF; has a mixed substrate with fibrosis often limited to the posterior wall. The electrical activity can be observed all over the LA, with frequent areas of fractionation and complex electrogram during AF.
Important comorbid conditions include underlying heart disease (eg, amyloidosis, mitral regurgitation, hypertrophic cardiomyopathy, congestive heart failure), diabetes, obesity, neuromuscular disease, prior stroke, and coronary artery disease, as well as older age. The presence of these comorbid conditions is important, since all of them increase the complexity of care, and many of these conditions themselves may lead to disease recurrence due to left atrial muscle stretching, oversynthesis of fibrotic tissue, extensive cellular loss, and extensive fibrosis.
‘The decision to ablate,” said Dr, Pappone, “should be based not on the response to external cardioversion but on the clinical environment of the AF disease” In the earliest phases, ablation of chronic AF can stop/delay progression. In patients with advanced anatomical disease with comorbidities, treatment of underlying disease, biventricular pacing, and left atrial appendage closure can be used to increase quality of life, improve hemodynamics, and reduce morbidity.
AF: From Prevention to Treatment
Samuel C. Dudley, Jr., MD, University of Illinois, Chicago, Illinois, USA, discussed the results of the Statins for Prevention of Atrial Fibrillation trial (StoP-AF; NCT00252967), which showed that while high-dose statins may have a systemic anti-inflammatory action, their use may not necessarily translate into a reduction in the recurrence of AF.
StoP-AF was a randomized, double-blind, placebo-controlled trial that investigated whether high-dose atorvastatin would maintain sinus rhythm after successful cardioversion in patients with persistent AF. Patients (64/524 subjects screened) with AF or atrial flutter (AFl) who required cardioversion were randomized to receive either atorvastatin 80 mg (n=33) or placebo (n=31). Cardioversion was performed, and statins were continued for 12 months or until AF recurred. Serum oxidative stress markers (ratios of oxidized-to-reduced glutathione and -cysteine, derivatives of reactive oxygen species, isoprostanes) and inflammatory markers (high-sensitivity C-reactive protein [hsCRP], interlukin-6 [IL-6], interlukin-1-beta [IL-1β], tumor necrosis factor-alpha [TNFα]) were measured at baseline and on follow-up. The primary study endpoint was the first ECG documentation of AF or AFl.
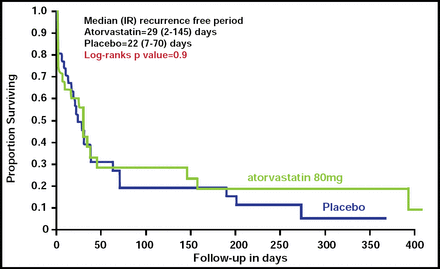
AF recurred in 22 (66.7%) of atorvastatin and 26 (83.9%) of placebo group subjects (p=0.2). There was no difference in the time to recurrence of AF between the two groups (Figure 1). Atorvastatin did not have a significant effect on any of the oxidative stress measures or on inflammation, as measured by IL-1β or TNF-α; however, IL-6 (adjusted OR, 0.59; 95% CI, 0.35 to 0.97) and hsCRP (adjusted OR, 0.59; 95% CI, 0.37 to 0.95) levels were significantly lower in the atorvastatin group at 1 month, as were cholesterol levels (p=0.03).
Time to Recurrence of AF.
Resproduced with permission from S. Dudley, Jr, MD.
Results from a subanalysis of data from the Randomized Evaluation of Long Term Anticoagulant Therapy trial (RE-LY; NCT00262600), presented by Ziad Hijazi, MD, Uppsala Clinical Research Center, Uppsala, Sweden, show that elevated cardiac troponin I (cTnI) levels are common in patients with AF and that troponin is a strong and independent predictor of stroke and other adverse outcome in this patient population. Thus, troponin may contribute to risk stratification when used with other clinical variables, such as the CHADS2-score.
In this substudy, investigators analyzed plasma concentrations of baseline cTnI in 6224 (of 18,113) RE-LY trial participants. Subjects were divided into 4 groups, based on their baseline cTnI levels:
-
Group 1: cTnI<0.01 ug/L (n=2681; 43%)
-
Group 2: cTnI=0.01 ug/L (n=1204; 19%)
-
Group 3: cTnI=0.02–0.03 ug/L (n=1725; 28%)
-
Group 4: cTnI≥0.04 ug/L (n=614; 10%)
Higher cTnI levels were significantly associated with the occurrence of the composite of stroke, systemic embolism, all-cause mortality, and also the composite of stroke, systemic embolism, pulmonary embolism, myocardial infarction, and cardiovascular death. Subjects with higher cTnI levels experienced significantly more major bleeding, suggesting that these patients had more comorbidities that placed them at higher risk for various complications. (Table 2).
Effect of cTnI Group.
‘Longer duration of early postoperative AF (POAF) is a strong independent predictor for the development of late AF,” said Rowlens M. Melduni, MD, Mayo Clinic, Rochester, Minnesota, USA, “and a postsurgical upstream therapeutic approach to prevent late recurrent AF should be considered in this patient population.”
Dr. Melduni presented the results of a study that examined the impact of POAF (AF ≤30 days) duration on the development of late AF in subjects (n=534) without prior history of AF, a pacemaker, or congenital heart disease who underwent CABG and/or valve surgery between 2000 and 2005. Subjects (mean age 65 ± 13.3 years; 70.4% men) were followed up to the last clinical visit, repeat surgery, or death for first documentation of AF after 30 days of surgery. The total follow-up period was 8 years (mean 4.4±2.5 years). The incidence of POAF during follow-up was 36.9% that lasted a median of 2 days. The average time from surgery to late AF was 2.5±2.3 years.
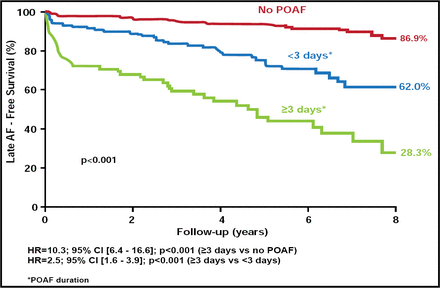
Over the 8 years of follow-up, significantly fewer subjects who experienced POAF remained free of late AF (48.8%) compared with those who did not experience POAF (86.9%; HR, 6.1; 95%CI, 4.0 to 9.3; p<0.001). Longer duration of POAF was also associated with an increased risk of late AF (POAF ≥3 days HR, 3.99; 95% CI, 2.29 to 6.95 vs POAF <3 days HR, 8.81; 95% CI, 4.91 to 15.80). Subjects with POAF duration ≥3 days were twice as likely to experience late AF compared with those with POAF of lesser duration and 8-fold more likely compared with those without POAF (Figure 2).
Relationship Between Duration of Early POAF and Occurrence of Late AF.
Resproduced with permission from R. Melduni, MD.
In multivariate analysis, after adjusting for age, gender, and clinical and surgical risk factors, other independent predictors of late AF were combined CABG and valve surgery (HR, 3.03; 95% CI, 1.81 to 5.09; p<0.001) and renal dysfunction (creatinine >2; HR, 2.33; 95% CI, 1.05 to 5.20; p=0.04).
The text above summarizes selected presentations from two sessions devoted to AF.
The editors would like to thank the many members of the European Society of Cardiology presenting faculty who generously gave their time to ensure the accuracy and quality of the articles in this publication.
- © 2010 MD Conference Express