Summary
Optimal antiplatelet therapy has been a source of contention, primarily due to conflicting trial data. There remain many unanswered questions regarding the role of platelet response testing, drug interactions, and bleeding risk in the clinical setting.
- Thrombotic Disorders
- Myocardial Infarction
Optimal antiplatelet therapy has been a source of contention, primarily due to conflicting trial data. There remain many unanswered questions regarding the role of platelet response testing, drug interactions, and bleeding risk in the clinical setting. These issues were the main topics of discussion at a high-profile session at the European Society of Cardiology Annual Meeting in Stockholm, Sweden.
There is a great deal of variability with regard to the therapeutic response of platelet inhibition. Therefore, individual risk profiles and the likelihood of a favorable response remain a concern when deciding upon a suitable antiplatelet regimen. Meinrad Gawaz, MD, Department of Cardiology, Eberhard Karls Universität, Tübingen, Germany, discussed the use of platelet response testing as a means to improve efficacy in patients with acute coronary syndromes (ACS) where antiplatelet treatment is indicated.
Antiplatelet therapy is often used at the time of and after percutaneous coronary intervention (PCI). A study by Geisler and colleagues found that low response to clopidogrel after stent implantation was associated with an increase in the risk of cardiac events and death and poor clinical prognosis (p<0.001) [Geisler T et al. Eur Heart J 2006]. Additionally, this nonresponsive status was associated with higher rates of early stent thrombosis (p=0.007), but late stent thrombosis (≥3 months post-PCI) did not appear to be influenced by postinterventional residual platelet aggregation (RPA) and was not prognostically predictive [Geisler T et al. Eur Heart J 2009]. In light of the fact that RPA serves as an independent risk factor for early stent thrombosis and is associated with poor clinical prognosis, it may be important to identify high-risk patients prior to treatment through the use of platelet response testing.
There are several factors that may contribute to therapeutic response, including cellular factors (ie, reduced metabolic activity, accelerated platelet turnover, and varying pathways as seen with clopidogrel), clinical factors (ie, compliance issues or comorbidities), genetic factors (ie, gene polymorphisms that result in reduced drug-specific responses), and pharmacokinetic factors (ie, poor absorption and drug-drug interactions) [Verstuyft C et al. Eur Heart J 2009]. Low response to clopidogrel and acetylsalicylic acid has been associated with inflammation, using the inflammatory biomarker C-reactive protein (CRP) [Müller K et al. Atherosclerosis 2010]. In the PREDICT study, nongenetic factors were found to be associated with increased RPA after PCI, thereby serving as valuable prognostic indicators [Geisler T et al. J Thrombosis & Haemostasis 2008]. Expanding on these findings, recent studies have determined that a combination of genetic and nongenetic factors may be the key to risk stratification and antiplatelet response prediction [Geisler T et al. Pharmacogenomics 2008; Hochholzer W et al. J Am Coll Cardiol 2010].
There are several studies currently underway that aim to investigate the utility of tailored antiplatelet therapy, based on prior risk stratification and predictive response measures. While the CYP2C19 genotype has been identified as a potential biomarker for therapeutic response, more study is needed before the predictive benefit can be established [Geisler T et al. Pharmacogenomics 2008; Hochholzer W et al. J Am Coll Cardiol 2010]. Tailoring antiplatelet treatment based on genetic and nongenetic factors shows promise and may be feasible in the future, but at present, there is insufficient clinical data to justify platelet testing in clinical practice, concluded Prof. Gawaz.
Stefano De Servi, MD, Ospedale Civile, Legnano, Italy, pointed out that the prevention of bleeding is an important goal; similar to the prevention of ischemic events, prevention in bleeding may result in a significant risk reduction for death, myocardial infarction (MI), and stroke. Bleeding is independently associated with poor clinical outcomes after intervention in patients with ACS. Therefore, the difficulty lies with balancing bleeding risk and the benefit of consistent platelet inhibition.
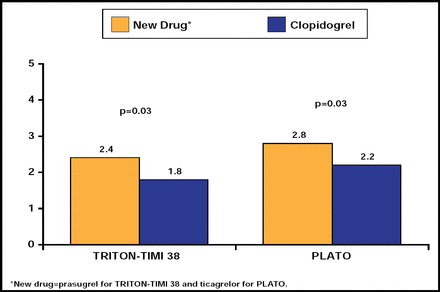
Antiplatelet agents have varying associated bleeding risks, and the individual risk should be considered prior to choosing a treatment strategy. A report by Sibbing and colleagues recently suggested a therapeutic window of P2Y12 receptor inhibition (such as that seen with prasugrel or ticagrelor) and associated risk reduction of bleeding and stent thrombosis [Sibbing D et al. J Am Coll Cardiol 2010]. The PLATO trial evaluated the effect of ticagrelor versus clopidogrel in patients with ACS and found that ticagrelor reduced the rate of death from vascular causes, MI, or stroke without increasing the rate of overall major bleeding. However, the rate of nonprocedure-related major bleeding was higher in the ticagrelor group than in the clopidogrel group (p=0.03) [Wallentin L et al. N Engl J Med 2009]. On comparison of data from the PLATO and TRITON-TIMI 38 trials, clopidogrel demonstrated more favorable rates of nonprocedure-related TIMI major bleeding compared with newer P2Y12 receptor inhibitors (ticagrelor and prasugrel; Figure 1) [Wallentin L et al. N Engl J Med 2009; Wiviott SD et al. N Engl J Med 2007]. Thus, the more potent antiplatelet agents may carry a higher bleeding risk; so, it is important to determine whether or not the benefit outweighs the cost. Risk stratification for bleeding should be included in the decision-making process.
Non-CABG-Related TIMI Major Bleeding in the TRITON-TIMI 38 and PLATO Trials.
Reproduced with permission from S. De Servi, MD.
The risk of gastrointestinal (GI) bleeding is also a concern with antiplatelet agents, but this risk may be reduced with concomitant proton pump inhibitor (PPI) therapy, particularly among patients on dual antiplatelet therapy and those with prior history of ulcer bleeding [Yeomans N et al. Am J Gastroenterol 2008; Lai K-C et al. Clin Gastroenterol Hepatol 2006]. However, there has been some debate as to the validity of the PPI+antiplatelet interaction. Tabassome Simon, MD, PhD, Université Pierre et Marie Curie, Saint Antoine Hospital, Paris, France, discussed recent data concerning PPI+antiplatelet agent use for the reduction of GI complications.
The existence of a PPI/clopidogrel interaction has received a great deal of attention in recent years. Pharmacologic studies demonstrated an in vitro interaction between PPIs and clopidogrel, which could theoretically increase cardiovascular (CV) risk [Simon T et al. N Engl J Med 2009; Verstuyft C et al. Eur Heart J 2009]. However, such concerns did not translate into clinical effects in the only double-blind, randomized, controlled clinical trial. In the COGENT Trial, PPIs did not dampen the efficacy of clopidogrel, but did prevent GI bleeding and other GI complications (the primary efficacy endpoint, rate of overt upper GI bleeding, was reduced with omeprazole compared with placebo; HR, 0.13; 95% CI, 0.03 to 0.56; p=0.001) the rate of the primary CV safety endpoint, the composite of death from CV causes, non-fatal MI, coronary revascularization, or ischemic stroke, was similar between the two groups (4.9% with omeprazole vs 5.7% with placebo; HR, 0.99; 95% CI, 0.68 to 1.44; p=0.96) [Bhatt DL et al. N Engl J Med 2010].
In addition, subanalyses from large clinical trials did not find a clinical interaction of PPIs with clopidogrel. Analysis of TRITON-TIMI 38 evaluating 13,608 patients with ACS who were randomized to receive either prasugrel (n=6813) or clopidogrel (n=6795), with 33% of patients (n=4529) on a PPI at randomization, found no association between PPI use and an increased risk of CV death, MI or stroke in patients who were being treated with clopidogrel (adjusted HR, 0.94; 95% CI, 0.80 to 1.11) or prasugrel (adjusted HR, 1.00;95% CI, 0.84 to 1.20) [O'Donoghue ML et al. Lancet 2009]. A meta-analysis by Hulot and colleagues found that the impact of PPI was influenced by baseline CV risk and appeared to primarily affect those at higher-risk [Hulot J-S et al. J Am Coll Cardiol 2010]. This therapeutic interaction may be more of a result of genetic polymorphisms (ie, the CYP2C19 genotype) than pharmacological interactions, but further investigation is required before the PPI debate can be settled [Simon T et al. N Engl J Med 2009].
Establishing an optimal antiplatelet regimen involves the consideration of many factors, including platelet response prediction, risk assessment, and drug interactions. Although incorporating the use of genotyping during the decision-making process may be helpful in the future, more studies are needed before implementing these procedures in clinical practice. For the time being, antiplatelet therapy should be based on evidence from large, randomized, clinical trials rather than retrospective subgroup analyses, and clinicians should consider the risk versus benefit when determining an appropriate treatment strategy for individuals for whom antiplatelet therapy is indicated.
- © 2010 MD Conference Express