Summary
Although the study failed to meet its primary endpoint, results from the ATOLL study indicate that the low-molecular-weight heparin enoxaparin may provide better clinical outcomes than unfractionated heparin in ST-elevation myocardial infarction patients who are undergoing primary percutaneous coronary intervention.
- Myocardial Infarction
- Cardiology Clinical Trials
- Interventional Techniques & Devices
Although the study failed to meet its primary endpoint, results from the ATOLL study, presented by Gilles Montalescot, MD, Pitié-Salpétrière Hospital, Paris, France, indicate that the low-molecular-weight heparin enoxaparin may provide better clinical outcomes than unfractionated heparin (UFH) in ST-elevation myocardial infarction (STEMI) patients who are undergoing primary percutaneous coronary intervention (PCI).
The Acute STEMI Treated with primary PCI and intravenous enoxaparin Or UFH to Lower ischemic and bleeding events at short- and Long-term follow-up study (ATOLL; NCT00718471) was an investigator-driven study that was designed to compare intravenous (IV) enoxaparin and IV UFH in patients who were undergoing primary PCI for STEMI. The study population comprised patients aged ≥18 years who presented within 12 hours of symptom onset. A total of 910 patients from 43 sites were randomly assigned to receive IV enoxaparin (n=450; 0.5 mg/kg; same dose with or without GPIIb/IIIa inhibitors) or IV-UFH (n=460; 50–70 IU/kg with GPIIb/IIIa inhibitors; 70–100 IU without GPIIb/IIIa inhibitors) before coronary angiography. All subjects received aspirin (160–500 mg/day according to local practice) and clopidogrel (300–900 mg as loading dose according to local practice). Approximately 18% of subjects were aged over 75 years.
The primary study endpoint was the composite of all-cause mortality at 30 days, complications of MI at 30 days (eg, resuscitated cardiac arrest, recurrent myocardial infarction/acute coronary syndrome (MI/ACS), urgent revascularization), procedure failure (eg, definite stent thrombosis), and non-CABG major bleeding during hospitalization. The main safety endpoint was major bleeding during hospitalization (STEEPLE definitions). The main secondary endpoint was the composite of all-cause mortality, recurrent MI/ACS, or urgent revascularization at 30 days.
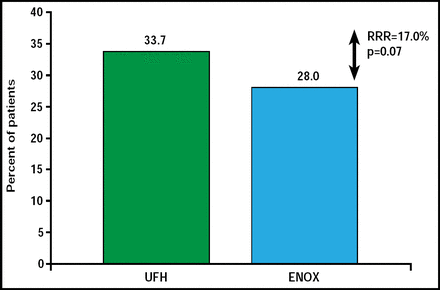
At 30 days, the primary endpoint of death, MI, procedural failure, or noncoronary artery bypass grafting major bleeding was reduced but did not reach statistical significance—28% of subjects received enoxaparin and 33.7% received UFH (relative risk reduction 17%; 95% CI, 0.68 to 1.01; p=0.07; Figure 1).
Primary Endpoint.
Reproduced with permission from G. Montalescot, MD.
For the main secondary endpoint, there was a significant (p=0.01) 41% reduction with enoxaparin (11.3% for UFH vs 6.7% for enoxaparin). All results for the other secondary endpoints favored enoxaparin IV (Table 1).
Other Secondary Endpoints.
The main safety endpoint occurred in 4.9% of patients who were on enoxaparin and 4.5% of patients who were on UFH (nonsignificant).
Commenting on the ATOLL study results, Harvey White, MD, Auckland City Hospital, Auckland, New Zealand, noted, “The ATOLL trial investigators have shown that enoxaparin is safe for patients undergoing primary PCI and likely has a clinically relevant effect in reducing ischemic complications compared with unfractionated heparin. They have moved us closer to the goal of further improving the outcomes of patients suffering an ST-elevation myocardial infarction.”
- © 2010 MD Conference Express