Summary
Results of the Systolic Heart Failure Treatment with the If Inhibitor Ivabradine Trial [SHIFT; ISRCTN70429960] indicate that when added to standard therapy, ivabradine reduced heart rate and improved outcomes in subjects with systolic heart rate as early as 3 months [Swedberg K et al. Lancet 2010].
- Heart Failure Cardiology Clinical Trials
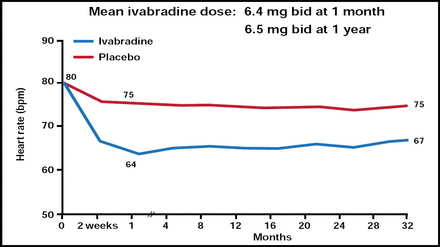
In patients with chronic heart failure (HF), elevated resting heart rate (HR) is a risk factor for adverse outcomes [Fosbøl EL et al. Int J Cardiol 2010]. Results of the Systolic Heart Failure Treatment with the If Inhibitor Ivabradine Trial (SHIFT; ISRCTN70429960), presented by Michel Komajda, MD, La Pitié-Salpétrière Hospital, Paris, France, indicate that when added to standard therapy, ivabradine reduced HR (Figure 1) and improved outcomes in subjects with systolic HF as early as 3 months [Swedberg K et al. Lancet 2010].
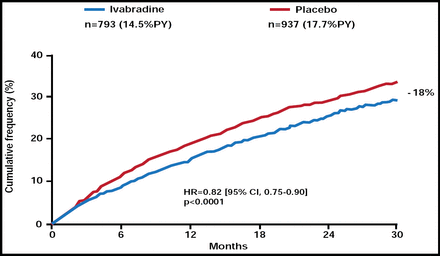
Primary Endpoint: Cardiovascular Mortality/Hospitalization for Worsening HF.
Reproduced from The Lancet, Volume 376, Issue 9744, Swedberg K, Komajda M, Bohm M et al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study, Copyright 2010, with permission from Elsevier.
All subjects had NYHA class II to IV HF, left ventricular ejection fraction ≤35%, and resting HR ≥70 beats per minute (bpm); received recommended HF therapy (∼90% on β-blockers; 56% at target daily dose); and had been hospitalized for worsening HF within the previous 12 months. Participants were randomly assigned to receive either ivabradine (n=3241; 5 mg bid, titrated to a maximum of 7.5 mg based on HR and tolerability) or placebo (n=3264). The primary outcome measure was a composite of cardiovascular (CV) mortality and hospitalization for worsening HF.
Median follow-up was 22.9 months. There was an 18% relative risk reduction [RRR] (absolute risk reduction of 4.2%) for the primary endpoint in patients who received ivabradine (HR, 0.82; 95% CI, 0.75 to 0.90; p<0.0001; Figure 1). The beneficial effect of ivabradine was driven mainly by a 26% RRR in hospitalizations for HF (HR, 0.74; 95% CI, 0.66 to 0.83; p<0.0001). Results were consistent among subjects, except that subjects with baseline HR ≥77 bpm had a greater reduction in the primary endpoint with ivabradine (p=0.029; Figure 2).
Mean Heart Rate Reduction.
Reproduced from The Lancet, Volume 376, Issue 9744, Swedberg K, Komajda M, Bohm M et al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study, Copyright 2010, with permission from Elsevier.
Deaths due to HF were significantly lower in subjects who received ivabradine versus placebo (HR, 0.74; 95% CI, 0.58 to 0.94; p=0.014). Although there were fewer CV (HR, 0.91; 95% CI, 0.80 to 1.03) and all-cause deaths (HR, 0.90; 95% CI, 0.80 to 1.02) in the ivabradine group, the differences were not significant (p=0.128 and p=0.092, respectively). There was a modest but significant (p=0.0003) improvement in NYHA class in the ivabradine group. Ivabradine was safe and well tolerated.
- © 2010 MD Conference Express