Renin-angiotensin-aldosterone system (RAAS) inhibitors reduce the risk of new-onset diabetes, microalbuminuria, and other adverse clinical outcomes in patients with hypertension. Matthew R. Weir, MD, University of Maryland School of Medicine, Baltimore, Maryland, USA, described new options for RAAS blockade in preventing end-organ damage in patients with elevated cardiovascular (CV) risk.
Several major clinical trials have demonstrated the benefits of angiotensin-converting enzyme inhibitors (ACE-Is) and angiotensin receptor blockers (ARBs) in slowing the progression of vascular disease and reducing markers of CV risk (Table 1) [Weir MR et al. J Clin Hypertens 2006]. Despite the use of traditional RAAS inhibitors, many hypertensive patients continue to have an increased risk for CV morbidity and mortality. Treatment with ACE-Is and ARBs only partially block RAAS, with a compensatory rise in other RAAS hormones, such as angiotensin I. Residual RAAS activation, as shown by elevated plasma renin activity (PRA) in patients on ACE-I or ARB therapy, may leave patients vulnerable to adverse outcomes.
Major Trials of RAAS Inhibition and End-Organ Protection.
Although investigators hoped that dual RAAS blockade with combination ACE-I/ARB therapy would provide greater CV risk reduction than single-agent RAAS inhibition, several trials have shown that this is not an effective strategy. In the Valsartan in Acute Myocardial Infarction (VALIANT) trial, the combination of valsartan and captopril failed to improve survival compared with captopril monotherapy (19.3% vs 19.5%; p=0.73), but increased the rate of discontinuation due to poor tolerability (19.0% vs 16.8%; p=0.007) [Pfeffer MA et al. N Eng J Med 2003]. In the Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial (ONTARGET), combination with telmisartan and ramipril did not reduce the primary endpoint of death from CV causes, MI, stroke, or heart failure hospitalizations compared with ramipril alone (16.3% vs 16.5%; HR, 0.99; 95% CI, 0.92 to 1.07) [The ONTARGET Investigators. N Eng J Med 2008].
The benefits of ACEIs and ARBs might be enhanced by addressing incomplete RAAS suppression with another form of RAAS blockade. Directly targeting renin is a new option for blocking RAAS at the point of activation without interfering with other metabolic pathways. Aliskiren, the first oral direct renin inhibitor, provides effective blood pressure control in patients with hypertension, reducing the systolic blood pressure by up to 15.7 mm Hg when given as high-dose (300 mg) monotherapy [Villamil A et al. J Clin Hypertens 2006].
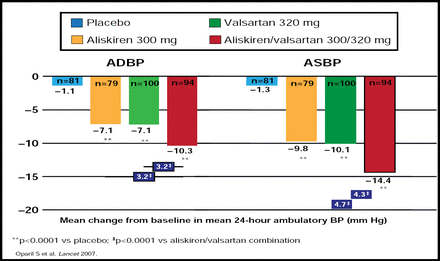
Treatment with aliskiren monotherapy effectively reduces PRA and, when used in combination with hydrochlorothiazide, blocks the rise in PRA that is seen during diuretic treatment (eg, mean reduction in baseline PRA, 46% to 64%) [Calhoun D et al. J Clin Hypertens 2006]. Therefore, aliskiren is an attractive candidate for RAAS inhibitor-based combination therapy. The potential benefits of dual RAAS blockade with aliskiren and an ARB were explored in a trial of 1797 patients with mild-to-moderate hypertension. After 8 weeks of treatment, combination therapy with aliskiren and valsartan provided significantly greater blood pressure reduction than either agent alone (Figure 1) [Oparil S et al. Lancet 2007].
Combination Therapy with Aliskiren and Valsartan Improves Blood Pressure Control Compared with Either Component as Monotherapy.
Reprinted from The Lancet, Vol. 370, Issue 9538, Oparil S et al, Efficacy and safety of combined use of aliskiren and valsartan in patients with hypertension: a randomised, double-blind trial, Pages 221–229, Copyright 2007, with permission from Elsevier.
The ASPIRE HIGHER clinical study program is currently underway to assess the incremental benefits of end-organ protection and CV event reduction with aliskiren alone or in combination with other antihypertensive agents, including RAAS inhibitors. Preliminary findings from the Aliskiren Observation of Heart Failure Treatment (ALOFT) trial suggest that aliskiren significantly reduces BNP levels compared with placebo (−61% vs −12.2%; p=0.016) in patients who are already on optimal heart failure therapy [McMurray JJV et al. ESC 2007]. Additional trials in the ASPIRE HIGHER program will evaluate direct renal inhibition in patients with hypertension, diabetes, and other CV risk factors. These studies may show whether combining a renin inhibitor with other RAAS blockers provides additional incremental benefits in blood pressure control and CV risk reduction.
- © 2010 MD Conference Express