Summary
Early treatment with the angiotensin receptor blocker (ARB) olmesartan significantly reduced the risk of developing microalbuminuria among patients with type 2 diabetes, according to new interim findings from the ongoing Randomized Olmesartan and Diabetes Microalbuminuria Prevention [ROADMAP] trial. However, olmesartan also appeared to increase the risk of cardiovascular mortality, raising concerns about the safety of ARBs in this patient population.
- Renal Disease
- Hypertension & Kidney Disease
- Diabetes Mellitus
- Diabetes & Kidney Disease Clinical Trials
- Hypertensive Disease
Early treatment with the angiotensin receptor blocker (ARB) olmesartan significantly reduced the risk of developing microalbuminuria among patients with type 2 diabetes, according to new interim findings from the ongoing Randomized Olmesartan and Diabetes Microalbuminuria Prevention (ROADMAP) trial. However, olmesartan also appeared to increase the risk of cardiovascular (CV) mortality, raising concerns about the safety of ARBs in this patient population.
The ROADMAP trial was designed to evaluate whether early intervention with an ARB prevented or delayed the onset of microalbuminuria (MAU), an early marker of renal disease and future CV events, in patients with type 2 diabetes. The multicenter randomized trial included 4449 men and women with type 2 diabetes, normal kidney function, and at least one additional CV risk factor, including hypertension. The mean baseline blood pressure (BP) level was 136/81 mm Hg.
Participants were randomly assigned to treatment with olmesartan 40 mg daily (n=2232) or placebo (n=2215). Patients were permitted to receive other antihypertensive medications during the study, but not other angiotensin-converting enzyme (ACE) inhibitors or ARBs. The primary endpoint was time to onset of MAU, and secondary endpoints included renal and CV events. Hermann Haller, MD, Hannover Medical School, Hanover, Germany, presented 48-month findings from the ongoing ROADMAP trial.
Treatment with olmesartan was associated with effective BP control. After 48 months, 78.2% and 71.3% of patients in the olmesartan and placebo groups, respectively, reached target BP levels of <130/81 mm Hg.
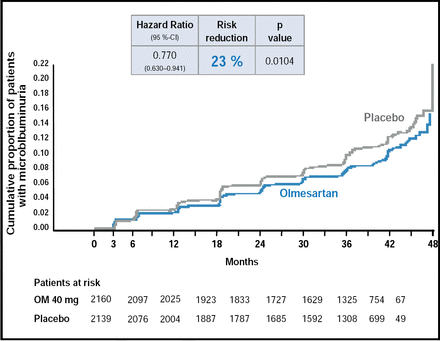
Patients in the olmesartan group were 23% less likely than those in the placebo group to develop microalbuminuria at 48 months (HR, 0.770; p=0.0104). The renoprotective benefit of olmesartan was independent of its effect on BP. MAU was less common in the olmesartan group after correcting for differences in diastolic BP (HR, 0.810; p=0.0398) and systolic BP (HR, 0.814; p=0.0451: Figure 1) between the ARB and placebo groups.
Time to First Occurrence of MAU.
According to a safety analysis, olmesartan showed no detrimental effects on renal outcomes at 48 months. The composite risk of CV morbidity and mortality was 4.3% in the olmesartan group and 4.2% in the placebo group (HR, 1.00; p=0.99). Fatal CV events were rare, but occurred more frequently in the olmesartan group (n=15) than in the placebo group (n=3; HR, 4.94; p=0.01). The risk of CV mortality with olmesartan relative to placebo was significantly increased only among patients with preexisting CVD (p=0.02). CV deaths in the olmesartan group were also associated with hypotension, occurring more frequently in patients with the lowest systolic BP levels and in those who experienced the greatest reduction in systolic BP.
On June 11, 2010, the U.S. Food & Drug Association (FDA) announced that it is reviewing interim safety data from ROADMAP and the Olmesartan Reducing Incidence of End Stage Renal Disease in Diabetic Nephropathy Trial (ORIENT), which also showed excess CV mortality with olmesartan compared with placebo. According to Prof. Haller, an observational follow-up study of ROADMAP is underway to further understand the long-term benefits of preventing the onset of MAU in patients with type 2 diabetes.
- © 2010 MD Conference Express