Summary
Real-time continuous glucose monitoring (RT-CGM) provides valuable information about glucose trends and patterns in diabetic patients. Recent trial data may provide evidence that supports the widespread use of RT-CGM in patients with type 1 diabetes mellitus. This article discusses recent landmark CGM studies and the clinical implications of these findings.
- Diabetes Mellitus
- Hyperglycemia/Hypoglycemia
Real-time continuous glucose monitoring (RT-CGM) provides valuable information about glucose trends and patterns in diabetic patients. This method of glycemic management offers the potential to improve bolus dosing and overnight control of insulin therapy among children and adults with type 1 diabetes mellitus (T1DM) through the use of hyper/hypoglycemia alarms and retrospective data that may assist clinicians in optimizing insulin-to-carbohydrate ratios and overnight basal rates. Recent trial data may provide evidence that supports the widespread use of RT-CGM in patients with T1DM.
William Tamborlane, MD, Yale University School of Medicine, New Haven, CT, presented findings from recent landmark CGM studies and discussed the clinical implications of these findings. In 2006, the GuardControl study revealed that CGM resulted in lower HbA1C levels and incremental improvement in diabetes control (from very poor to poor). However, the study was relatively small (n=162 patients) and evaluated outcomes over a short period of time (12 weeks) [Deiss D et al. Diabetes Care 2006].
Recently, the Juvenile Diabetes Research Foundation CGM study explored the safety and efficacy of CGM in patients with T1DM and HbA1C levels of ≥7.0% and <7.0%. The study involved two trial arms. In the first part of the study, patients were stratified according to age group (age 8 to <15 years, 15 to <25 years, and ≥25 years) and randomized to receive either RT-CGM or standard self-monitoring of blood glucose. There were approximately 110 patients in each age group. The primary outcome was difference in HbA1C from baseline to 6 months. The second arm of the study included 129 patients with baseline HbA1C of <7.0%. Approximately 50% of patients in this cohort were <25 years old. All patients wore blinded CGM devices at baseline, and patients in the control group wore blinded CGM devices at Weeks 13 and 26. The primary outcome was the difference in frequency of sensor glucose levels <70 mg/dL.
At 6 months, patients with baseline HbA1C levels > 7.0% who received RT-CGM within the ≥25 years age group demonstrated more favorable improvement in HbA1C levels compared with control, and this improvement was sustained for 12 months. There was no difference between RT-CGM and control in patients under the age of 25 years. Change in HbA1C correlated with the frequency of CGM use. In well-controlled patients with baseline HbA1C levels <7.0%, sensor glucose levels of ≤70 mg/dL occurred more often in the control group than in the CGM group (p=0.05 for Weeks 13 and 26). At 26 weeks, 88% of patients in the RT-GCM group were able to maintain optimal A1C levels (<7.0%) versus 63% in the control group (p<0.001).
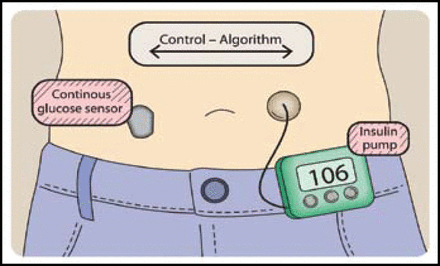
The closed loop CGM, often termed the “artificial pancreas,” consists of a continuous glucose monitor, insulin infusion pump, and a control algorithm that delivers insulin according to real-time glucose readings. This closed loop control system includes a self-correcting feedback loop (Figure 1). Roman Hovorka, PhD, University of Cambridge, Cambridge, United Kingdom, discussed the progress that is being made concerning the overnight closed-loop approach. Postprandial glucose control remains a challenge, and 55% to 75% of severe hypoglycemia episodes occur at night [DCCT Research Group. Am J Med 1991; Davis et al. Diabetes Care 1997]. Therefore, studies have been designed to evaluate the effect overnight closed loop devices on the incidence of nocturnal hypoglycemia and euglycemic achievement in patients with T1DM.
The Artificial Pancreas.
Reproduced with permission from R. Hovorka, MD, PhD.
In a secondary pooled-data analysis of a Phase II, randomized, crossover trial by Hovorka and colleagues that compared closed loop and continuous subcutaneous insulin infusion therapy, closed loop therapy was associated with increased time in target range (p=0.002) and reduced time for which glucose concentrations were ≤3.90 mmol/L (p=0.03) in children and adolescents with T1DM. There were 9 hypoglycemia events (plasma glucose concentration <3.0 mmol/L) that were observed in the continuous subcutaneous insulin infusion arm versus no events in the closed loop arm [Hovorka R et al. Lancet 2010]. Based on these results, investigators concluded that closed loop systems could reduce the risk of nocturnal hypoglycemia in children and adolescents with T1DM.
Home study using simulations may be the next step in closed loop evaluation. Wilinska and colleagues have created an algorithm to assess the risk of hypoglycemia and hyperglycemia in the home setting using simulations [Wilinska ME et al. J Diabetes Sci Technol 2009; Kovatchev et al. J Diabetes Sci Technol 2009]. These algorithms can be tested on in silico patients prior to clinical study and eliminates the need for animal testing. The ultimate goal is to perfect closed loop technology, thus normalizing glucose levels through the use of existing technologies, improved glucose sensors, multihormone systems, and more efficient insulin absorption techniques. There are many studies underway that may provide evidence for the use of closed loop systems in the near future.
Aaron Kowalski, PhD, Juvenile Diabetes Research Foundation International, New York, NY, discussed current areas of research focus that are related to the development of the artificial pancreas. The main priorities at present appear to be the following: control to range (a system that would minimize hypoglycemic and hyperglycemic fluctuations), nocturnal pump shutoff (a system that would include a nocturnal hypoglycemia prediction tool that triggers insulin delivery suspension for up to 2 hours), overnight closed loop control (a system that would control blood glucose while sleeping), and multihormone (a bihormonal system with insulin and glucagons or other hormones). While the algorithmic approach has been advancing artificial pancreas technology at a rapid pace, the artificial pancreas clinical pathway is still being developed.
The level of automation that is required from the artificial pancreas will likely emerge in stages, with the aim of restoring normal physiology in patients with T1DM. Current CGM devices can potentially minimize hypoglycemic events through the use of algorithmic controls. More efficient insulin kinetics and the incorporation of other hormones within these systems may provide superior devices in the future. As this technology progresses, so must the system components (sensors, algorithms, and pumps) in order for the concept of a fully automated system to become a reality.
Marilyn Ritholz, PhD, Joslin Diabetes Center, Boston, MA, pointed out that there are barriers beyond the technology of CGM. Psychosocial factors may play a role in CGM success. Using a sample from the JDRF cohort (n=20), Ritholz and colleagues assessed potential barriers to CGM success, based on patient demographics and interviews [Ritholz M et al. in press]. While body image concerns occurred in both responders and nonresponders, an increase in self-consciousness, excessive focus on diabetes, and a sense of feeling robotic or different was associated with CGM use. Other barriers to successful CGM use include more frequent use of emotion-based versus self-controlled coping with frustrations, less family involvement, and less use of retrospective analysis of CGM data.
It is important to be aware of the patient's perspective, related to CGM use. The patient experience may dictate the success or failure of this technology.
RT-CGM shows promise for the future. Though the artificial pancreas technology is not yet a reality, development appears to be headed in the right direction. CGM may allow patients to achieve and sustain better glucose control and help prevent hypoglycemic and hyperglycemic nocturnal events. It also provides valuable information regarding glucose trends and patterns for physicians who are treating T1DM patients. Increased safety and effectiveness remain priorities with the development of this innovative technology, and the fully automated artificial pancreas appears to be an attainable goal.
- © 2010 MD Conference Express